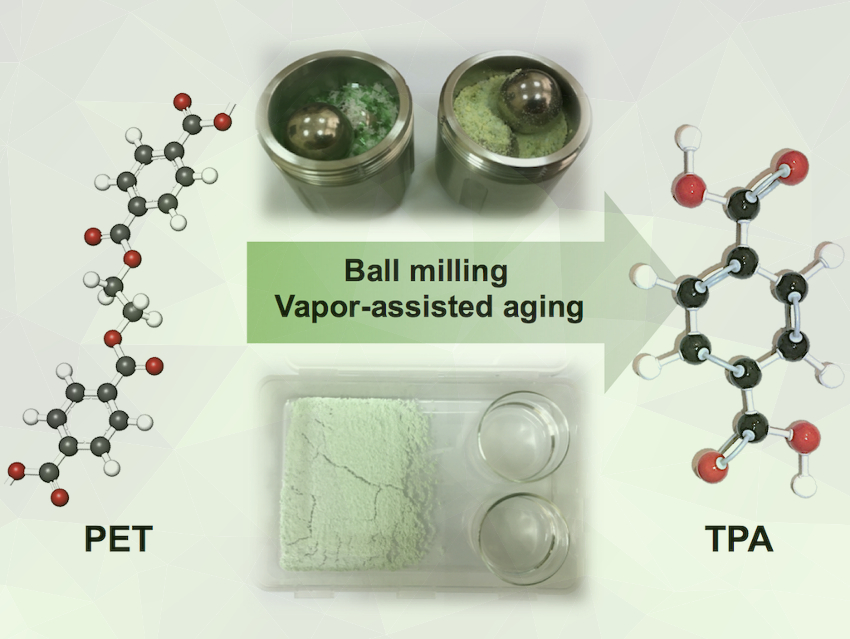
Polyethylene terephthalate (PET) is a synthetic polyester widely used in the production of soft-drink bottles and textile fibers. PET is a thermoplastic made of repeating units of terephthalic acid (TPA) and ethylene glycol (EG), linked together via an ester bond. The ester linkage between TPA and EG units can be cleaved by hydrolysis to transform PET waste back into its monomer constituents. Current chemical methods of PET recycling require the use of organic solvents at high temperature and pressure to achieve depolymerization into monomer derivatives in practical yields.
Vjekoslav Štrukil, Ruđer Bošković Institute, Zagreb, Croatia, found that the challenging breakdown of waste PET under ambient temperature and pressure can be achieved by mechanochemical ball milling or vapor-assisted aging. First, a mixture consisting of pre‐milled waste PET and sodium hydroxide pellets was ball-milled. The crude mixture was then suspended in distilled water, the unreacted PET removed by filtration, and the filtrate was acidified with diluted HCl, resulting in the precipitation of TPA monomer in yields of up to 99 %.
Excellent TPA yields were also achieved by aging pre-milled solid mixtures of PET and a base in a humid environment or in the presence of alcohol vapors. This approach represents an even milder route to PET depolymerization with conversions up to 99 %. Both methods perform well on the gram-scale, showing their promise for a green and sustainable high-yielding production of TPA by hydrolysis of post-consumer PET in the solid state.
- Highly efficient solid‐state hydrolysis of waste polyethylene terephthalate by mechanochemical milling and vapour‐assisted aging,
Vjekoslav Štrukil,
ChemSusChem 2020.
https://doi.org/10.1002/cssc.202002124