The interhalogen BrCl exists in an equilibrium with Br2 and Cl2, with a ratio of 60 % BrCl, 20 % Br2, and 20 % Cl2. This hampers the stoichiometric use of neat BrCl as a reagent due to undesired side products. However, BrCl can be stabilized by halogen bonding interactions. The addition of a halogen bond acceptor (e.g., Cl–) leads to a drastic shift of the equilibrium to the BrCl side.
Sebastian Riedel, Free University of Berlin, Germany, and colleagues have used this effect to generate BrCl in the form of ionic liquids. The desired reactive ionic liquids are obtained by condensing Cl2 and Br2 onto a chloride salt such as [NEt4]Cl. Warming up the reaction mixture to room temperature then leads to the formation of BrCl, which immediately reacts with the chloride to give the desired polyinterhalide species. Using this approach, a set of BrCl polyinterhalides of the type [Cl(BrCl)n]– (n = 1–6) was synthesized. All compounds were characterized by single-crystal X-ray diffraction, Raman spectroscopy, and quantum-chemical calculations.
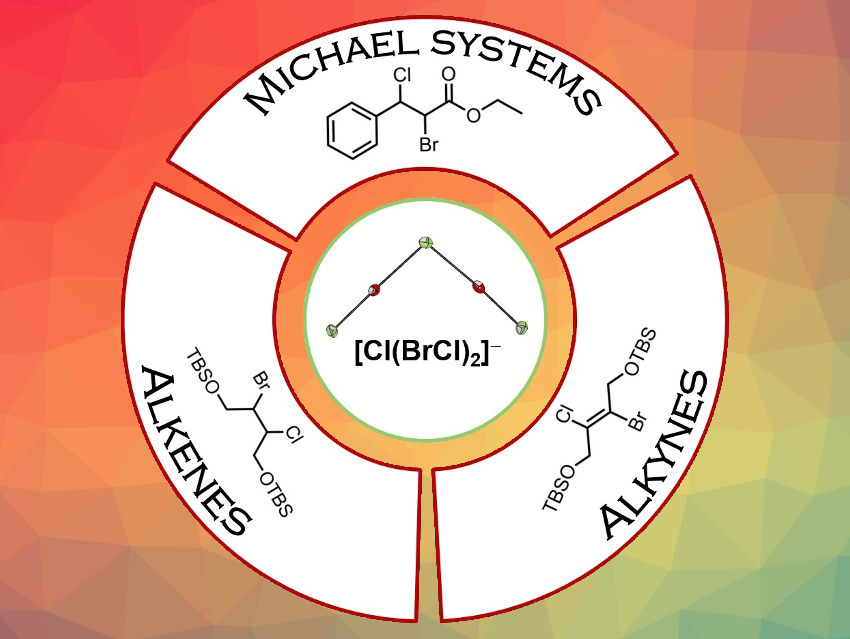
This stabilization of BrCl in the form of safer and easier-to-handle ionic liquids is useful for applications such as interhalogenation reactions of alkenes, alkynes, or Michael systems (pictured). In addition, the work has expanded the structural diversity of polyhalogen compounds.
- In Situ Synthesis and Applications for Polyinterhalides Based on BrCl,
Benjamin Schmidt, Sebastian Ponath, Johannes Hannemann, Patrick Voßnacker, Karsten Sonnenberg, Mathias Christmann, Sebastian Riedel,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.202001267