One of the main challenges for manned Mars expeditions is the need for sufficient oxygen and fuel. Scientists have developed a water electrolyzer that produces pure oxygen under Mars-like conditions. According to the study, this electrolyzer operates at lower input power than the carbon dioxide electrolyzer NASA plans to test on its current Mars mission. This new water electrolyzer could make use of the water reservoirs existing beneath the surface of the red planet.
Oxygen Production on Mars
Once they have arrived on Mars, humans will need oxygen to breathe, but they will also need large quantities of oxygen fuel to propel their rockets back to Earth. There is only a very small amount of oxygen in the thin Martian atmosphere, which contains 96 % of CO2, the remainder mostly consisting of argon and nitrogen. Consequently, the spacecrafts must either bring the oxygen with them or generate it on site.
To explore oxygen generation on the planet, NASA has included a carbon dioxide electrolyzer in its current 2020 Mars mission. This Mars Oxygen In-Situ Resource Utilization Experiment (MOXIE) is designed to split CO2 from the Martian atmosphere into O2 and CO. The process takes place on a stack of electrodes, which are heated to several hundred degrees Celsius. The CO side product is released as waste. In contrast, water electrolysis not only produces oxygen, but it also generates hydrogen, which is a valuable fuel.
Brine Water on Mars
NASA’s Phoenix lander and several Mars orbiters have found strong indications that liquid water exists on Mars. Large ponds are predicted to lie beneath the Martian surface, filled with brine and prevented from freezing by their high perchlorate concentration. Scientists estimate that these so-called regolithic brines stay liquid down to temperatures of –60 °C.
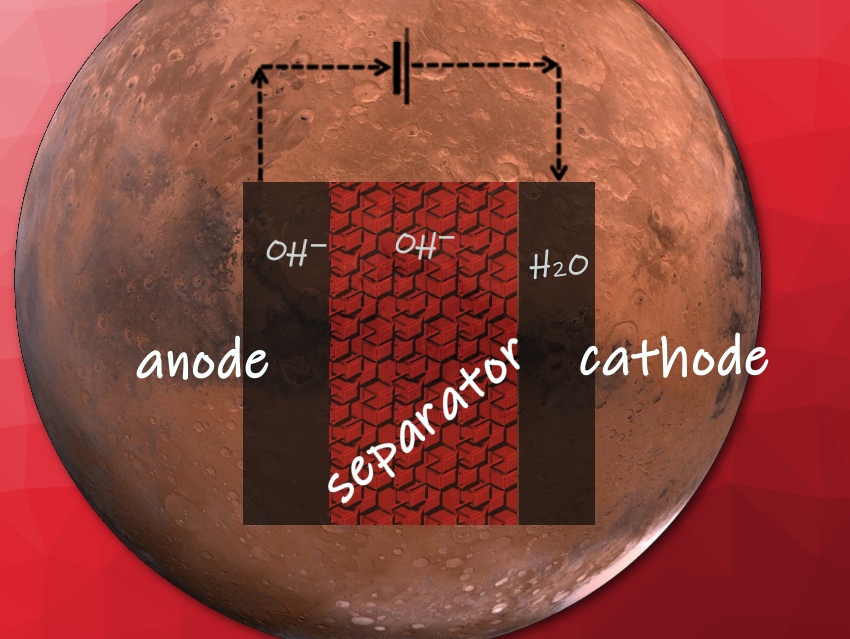
Water electrolysis normally requires an aqueous electrolyte containing only a small concentration of non-interfering salts, just to provide ion conductivity. Martian brines, in contrast, are full of salts, and the extreme cold on the planet would choke off any kinetics of the electrodes.
Mimicking the Brine Water Conditions on Mars
A variety of electrocatalysts exist for the oxygen evolution reaction (OER), most of which are metal oxides. Vijay K. Ramani and colleagues from the Department of Energy, Environmental & Chemical Engineering at Washington State University in St. Louis, MO, USA, tested the pyrochlore compound Pb2Ru2O7–δ under conditions mimicking those found on Mars. Pyrochlores, with the general formula A2B2O7 (A and B being metals), are known for their fast ionic conductivity and usefulness in diverse technical applications.
The researchers assembled the electrolyzer using a glassy-carbon-supported Pb2Ru2O7−δ anode, a carbon-supported platinum cathode, a commercial anion-exchange membrane separator, and a simulated regolithic brine consisting of 2.8 molar magnesium perchlorate solution saturated with CO2. A bath of dry ice in an ethylene glycol–ethanol mixture kept the temperature at −36 °C to model the average temperature on the surface of Mars.
The researchers found the electrolyzer produced pure oxygen and hydrogen with an estimated energy efficiency of up to 60 %. For comparison, current water electrolyzers operate with efficiencies of 60–80 %, but this is under the gentle conditions found on Earth.
Comparison to MOXIE
NASA expects its MOXIE to produce about 10 to 20 grams of oxygen per hour, which is about a third of the amount a human being needs to breathe when resting. MOXIE is located inside the Perseverance rover that is currently on its way to the red planet. It is the size of a car battery, with a mass of 17 kg. To support manned missions, NASA says it will have to be 100 times larger.
To compare the novel brine electrolyzer with MOXIE, the researchers calculated how large and heavy their device would have to be to match the oxygen generation ability of MOXIE. Working under the simulated Martian surface conditions, they found that the brine electrolyzer would be smaller, lighter, and would produce more than 25 times the amount of oxygen that MOXIE does for the same input power.
“Or, to put it another way, our system consumes 25 times less power than MOXIE, for the same oxygen production rate,” the scientists said. In addition, their device produces hydrogen fuel instead of toxic CO, and it works at average Martian temperatures, with no heating required. Therefore, the team advocates considering their electrolyzer design for the next Mars mission. In the meantime, they plan to explore its use for fuel generation from seawater or other salty liquids.
- Fuel and oxygen harvesting from Martian regolithic brine,
Pralay Gayena, Shrihari Sankarasubramaniana, Vijay K. Ramani,
Proc. Natl. Acad. Sci. 2020.
https://doi.org/10.1073/pnas.2008613117