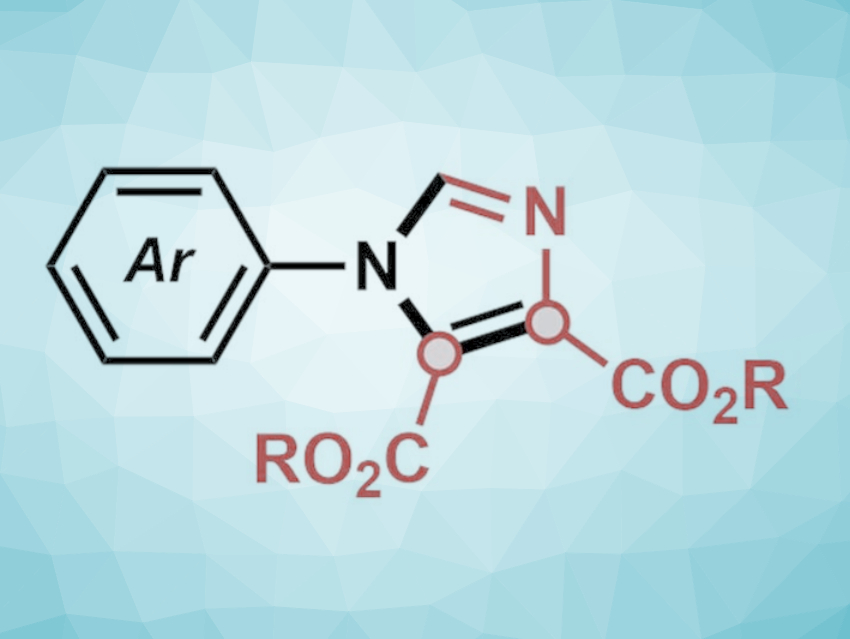
Polysubstituted imidazoles, five-membered nitrogen-containing heterocyclic compounds, are found in various bioactive natural products and pharmaceuticals. Nitrones (N-oxides of imines) and isocyanides are both commonly used as commercially available starting materials for the construction of nitrogen-containing compounds. However, the cross-reaction between nitrones and isocyanides has only been used in a handful of synthetic protocols.
Jian‐Quan Liu, Jiangsu Normal University, China, and KTH Royal Institute of Technology, Stockholm, Sweden, Markus D. Kärkäs, KTH Royal Institute of Technology, Xiang‐Shan Wang, Jiangsu Normal University, and colleagues have developed a silver-mediated method for annulations between isocyanoacetates and nitrones, providing access to 1,4,5-trisubstituted imidazoles (pictured below). The team used AgOTf as the catalyst, KOTf as an additive, and toluene as the solvent.
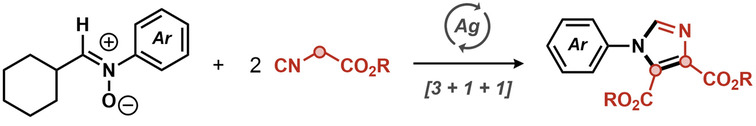
With this approach, various trisubstituted imidazoles with common functional groups could be synthesized in good to high yields. The team proposes that the reaction proceeds via a formal [3+2] cycloaddition, ring‐opening, dealkylation, and cyclization‐oxidation process.
- Silver‐Catalyzed [3+1+1] Annulation of Nitrones with Isocyanoacetates as an Approach to 1,4,5‐Trisubstituted Imidazoles,
Lanlan Lv, Yan Chen, Andrey Shatskiy, Jian-Quan Liu, Xiaoyi Liu, Markus Kärkäs, Xiang-Shan Wang,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202001536