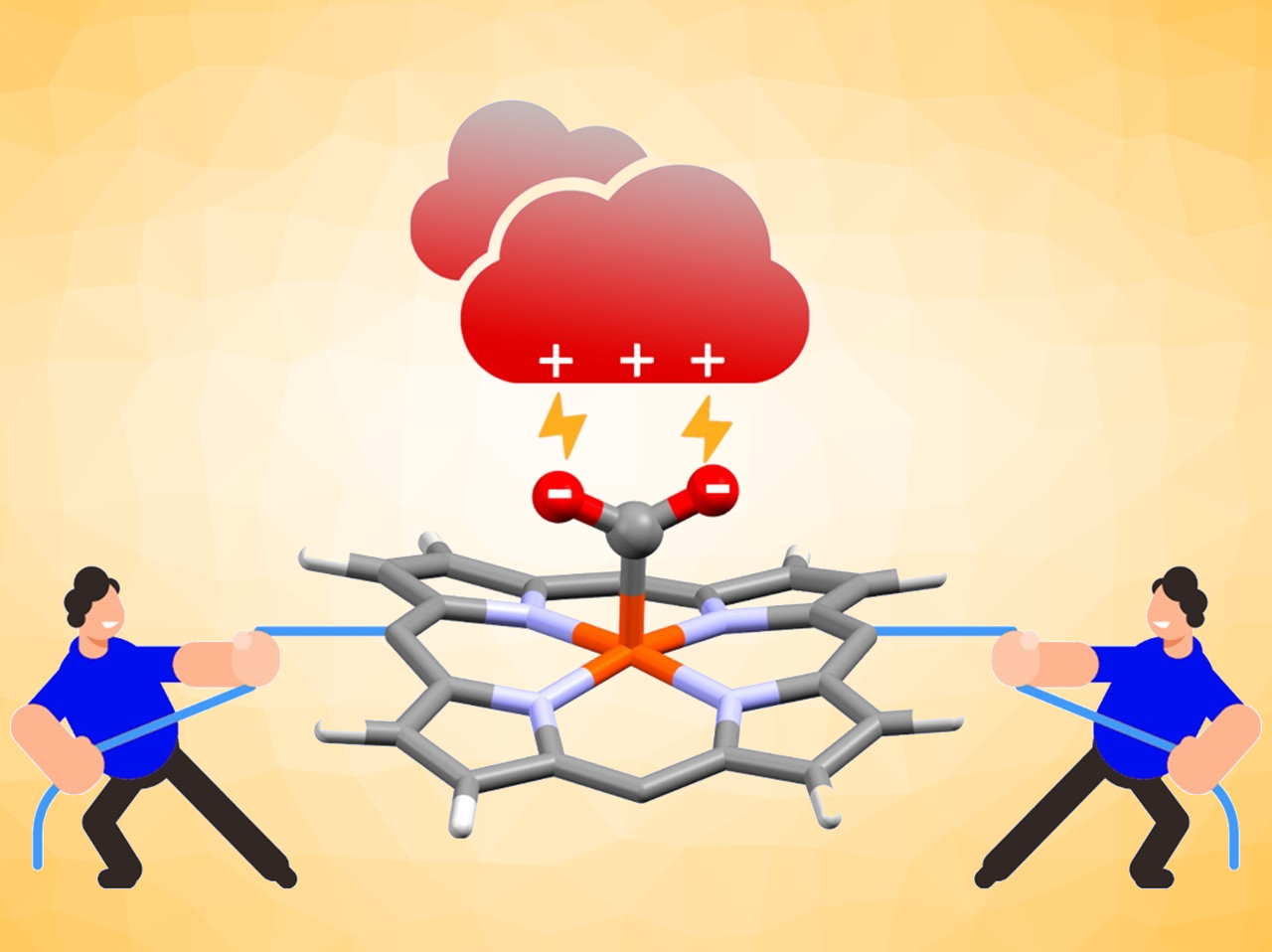
In enzymatic reactions, electrostatic interactions can play a major role in the activation of substrates. This can also inspire the design of molecular catalysts for useful reactions. The CO2 reduction reaction, for example, could be important to mitigate climate change. Metalloporphyrins are promising electrocatalysts for this reaction.
Zakaria Halime, Ally Aukauloo, Université Paris-Saclay, France, and colleagues have developed substituted iron porphyrins with cationic imidazolium groups (pictured below) as catalysts for the CO2 reduction reaction. The team designed the iron porphyrins to stabilize a proposed [Fe−CO2] intermediate (pictured above) through electrostatic interactions with the cationic groups. The catalysts were prepared from aminophenylporphyrins via a reaction with 3‐(chloromethyl)benzoyl chloride to create a linker, followed by a functionalization with N‐methylimidazole to introduce the desired methyimidazolium groups.
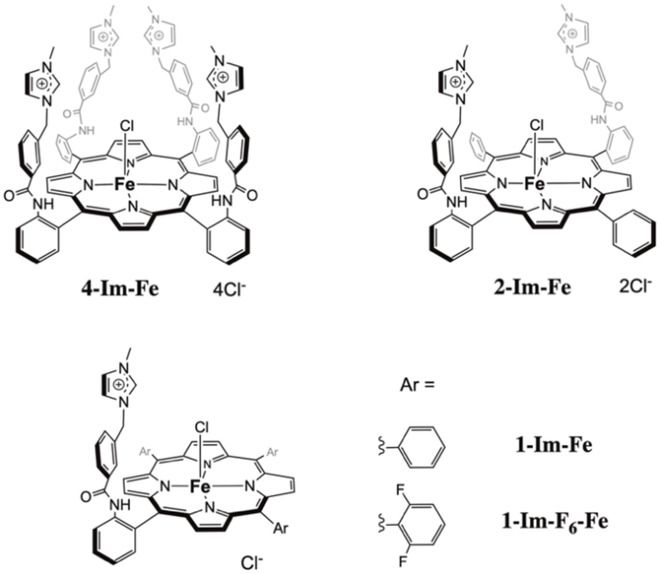
The team found that the number of cationic groups influences the catalytic performance. They observed an increase of the reaction overpotential and the turnover frequency (TOF) with decreasing numbers of methylimidazolium groups. They also found that these through-space electrostatic effects can outperform the “classical” through-bond electronic effects strategy using electron-withdrawing or -donating substituents (e.g., fluorine atoms).
- Through‐Space Electrostatic Interactions Surpass Classical Through‐Bond Electronic Effects in Enhancing CO2 Reduction Performance of Iron Porphyrins,
Ally Aukauloo, Asma Khadhraoui, Philipp Gotico, Winfried Leibl, Zakaria Halime,
ChemSusChem 2021.
https://doi.org/10.1002/cssc.202002718