Linking molecular components through amide bonds is one of the most important reactions in research and the chemical industry. Rajavel Srinivasan, Tianjin University, China, and colleagues have developed a new type of reaction for making amide bonds. Called an ASHA ligation, this reaction is fast, efficient, works under mild aqueous conditions, and is broadly applicable.
Making Amides
Amide bonds are the bond between a carbonyl carbon (C=O) and an organic nitrogen atom. Amide bonds link individual amino acids together into proteins and bind monomers into polyamide plastics like perlon and nylon. Many life-saving medicines such as taxol, lipitor, and penicillin, as well as agrochemicals, biological conjugates, natural substances, and other products contain amide bonds. In addition to the classical method of production, i.e., the reaction of an acid group (–COOH) with an amino group (–NH2), a variety of different reactions have been developed for the formation of amide bonds. However, many of them are not broadly applicable due to a lack of chemoselectivity. They also require specialized coupling agents. New types of reactions are in demand.
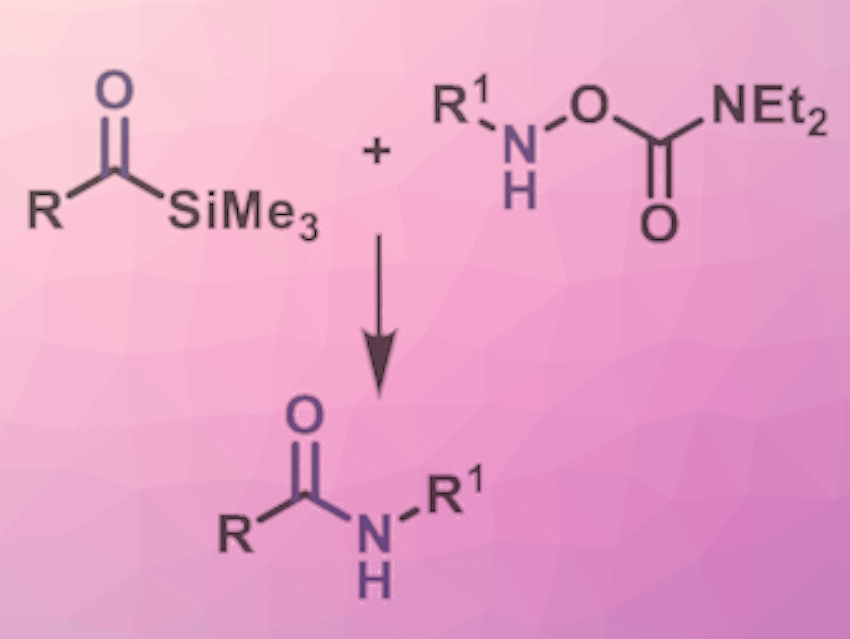
The team was searching for an efficient, sustainable method that starts with easily accessible materials and works fast. They chose a reaction between acylsilanes and hydroxylamines (a class of organic nitrogen compounds with an –N–O– linkage). Acylsilanes are a class of organic silicon compounds with a general structural formula of R(CO)-SiR3. Their reactivity often differs completely from that of the related ketones. Although their chemistry is well known, they have previously rarely been used in the field of biomedical chemistry.
ASHA Ligation
The reaction of AcylSilanes with HydroxylAmines is called an ASHA ligation, for short. The driving force appears to be the intramolecular migration of a silyl group from a carbon atom to an oxygen atom (Brook rearrangement), in which a strong Si–O bond is formed.
The researchers successfully tested the ligation with a variety of different molecular building blocks, including pharmaceutical agents, peptides, natural products, and other biologically active compounds. The reaction is chemoselective and works under mild conditions in an aqueous environment. It produces high yields in the majority of cases and is tolerant toward most functional groups. New variants of the ASHA ligation are in development for peptide synthesis, as this did not work well so far.
However, the simplicity and efficiency of the ASHA ligation should open pathways to new approaches in medicinal chemistry and chemical biology, such as fragment-based drug development, in which active substances are built up successively from small fragments.
- Chemoselective Amide‐Forming Ligation Between Acylsilanes and Hydroxylamines Under Aqueous Conditions,
Xingwang Deng, Guan Zhou, Jing Tian, Rajavel Srinivasan,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202012459