Organic micropollutants such as dyes, pesticides, hormones, and pharmaceuticals can be harmful to human health and aquatic ecosystems. Macrocycle-containing porous organic polymers (POPs) can be used for host-guest encapsulation and have high surface areas, which makes these materials attractive for use in water treatment to remove pollutants.
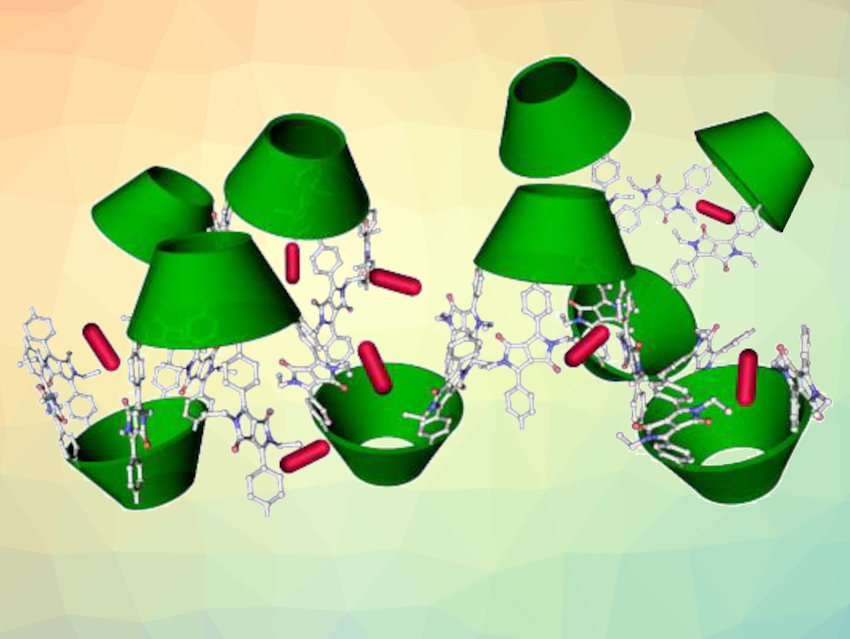
Xiaofan Ji, Huazhong University of Science and Technology, Wuhan, China, Hongyu Wang, Shanghai University, China, Jonathan L. Sessler, Shanghai University and The University of Texas at Austin, USA, and colleagues have developed a POP based on the bowl-shaped receptor calix[4]pyrrole. The compound (pictured) was obtained via a Sonogashira coupling of α,α,α,α‐tetraalkynyl calix[4]pyrrole with diketopyrrolopyrrole (DPP) monomers.
The polymer’s structure was further modified by converting tert-butyl ester substituents at the DPP units to carboxylic acid or carboxylate groups to make it less hydrophobic. Treating the polymer with trifluoroacetic acid (TFA) removed the tert‐butyl ester groups to give free carboxylic acid groups, followed by treatment with aqueous sodium hydroxide at room temperature to give the anionic, carboxylate-functionalized polymer.
The carboxylate-modified, anionic POP showed excellent absorption of cationic micropollutants, with capacities as high as 454 mg g–1 for methylene blue, 344 mg g–1 for the herbicide paraquat, and 495 mg g–1 for the herbicide diquat. According to the researchers, these uptake values are significantly higher than those of most synthetic adsorbent materials reported to date.
- Calix[4]pyrrole-Crosslinked Porous Polymeric Networks for Micropollutant Removal from Water,
Jonathan L. Sessler, Xiaohua Wang, Linhuang Xie, Kunhua Lin, Weibin Ma, Tian Zhao, Xiaofan Ji, Niveen M. Khashab, Mram Alyami, Hongyu Wang,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202016364