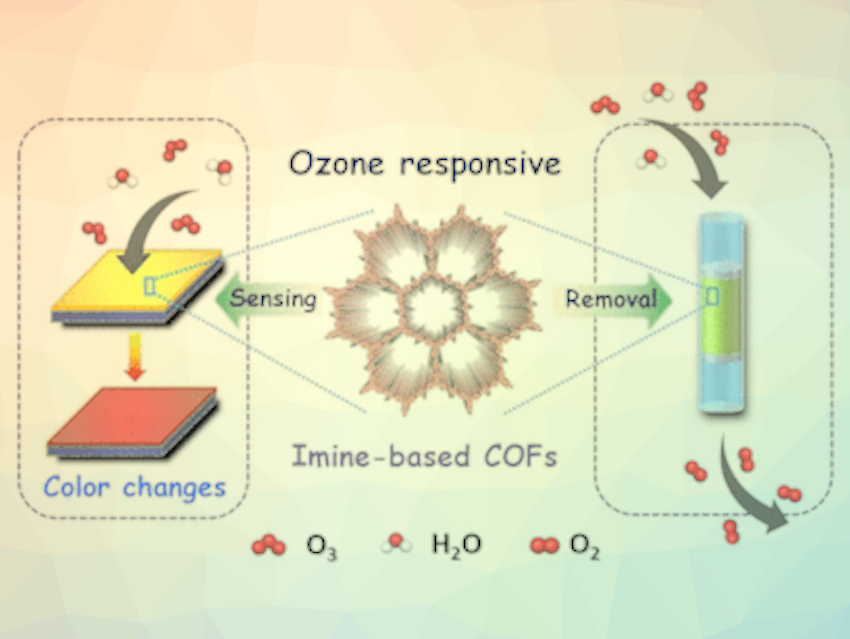
Ozone is a problematic air pollutant that causes serious health problems. Zhenjie Zhang, Nankai University, Tianjin, China, and colleagues have developed a material that not only quickly and selectively indicates the presence of ozone, but also simultaneously renders the gas harmless. The porous “2-in-one systems” also function reliably in very humid air.
Rapid Ozone Detection
Ozone (O3) can cause health problems such as difficulty breathing, lung damage, and asthma attacks. Relevant occupational safety regulations, therefore, limit the concentrations of ozone allowable in the workplace. Previous methods for the detection of ozone, such as those based on semiconductors, have a variety of disadvantages, including high power consumption, low selectivity, and malfunction due to humid air. Techniques aimed at reducing the concentration of ozone have thus far been based mainly on activated charcoal, chemical absorption, or catalytic degradation.
The researchers set themselves the goal of developing a material that can both rapidly detect and efficiently remove ozone. Their approach uses materials known as covalent organic frameworks (COFs). COFs are two- or three-dimensional organic solids with extended porous crystalline structures; their components are held together by strong covalent bonds. COFs can be tailored to many applications through the selection of different components.
Efficient Degradation Using COFs
The researchers selected easily producible, highly crystalline COFs made of aromatic ring systems. The individual building blocks are bound through imine groups. These are at the center of the action.
The imine COFs indicate the presence of ozone through a rapid color change from yellow to orange-red, which can be seen with the naked eye and registered by a spectrometer. Unlike many other detectors, the imine COF also works very reliably, sensitively, and efficiently at high humidity and over a wide temperature range. In the presence of water, the water molecules will preferentially bind to the imine groups. Consequently, the researchers assert, a hydroxide ion (OH−) is released, which reacts with an ozone molecule. The positively charged hydrogen atom remains bound to the imine group, causing the color change.
If more ozone than water is present (or the ozone-laden air is fully dry), the excess ozone binds to the imine groups and splits them. Each imine group degrades two molecules of ozone. This also causes a color change and the crystalline structure slowly begins to collapse. The imine COF, thus, does not just detect the ozone, but also reliably and efficiently breaks the harmful gas down. This makes it more effective than many of the traditional materials employed for this purpose.
- Rational Fabrication of Crystalline Smart Materials for Rapid Detection and Efficient Removal of Ozone,
Dong Yan, Zhifang Wang, Peng Cheng, Yao Chen, Zhenjie Zhang,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202015629