There is an increasing demand for high-capacity lithium-ion batteries. All-solid-state batteries can use a lithium-metal anode and maximize the energy density. One of the key challenges in this context is the efficient manufacturing of solid electrolytes. Lithium thiophosphates, for example, provide high conductivities and good processability. One example is Li3PS4, which is typically prepared from Li2S and P4S10. Splitting the adamantane-cage-like precursor P4S10 has been proposed to be the rate‐limiting reaction step.
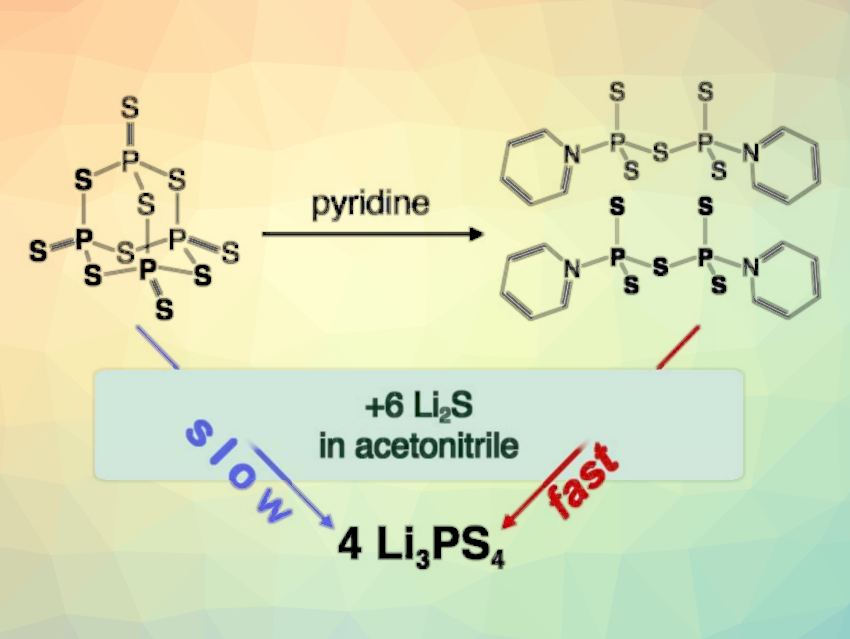
Wolfgang Zeier, University of Münster, Germany, and colleagues have developed an approach in which P4S10 is readily and cleanly split using pyridine. The resulting stable pyridine–P2S5 complexes were then used in the solvent-based synthesis of the electrolyte Li3PS4. The team mixed P4S10 powder with pyridine and obtained py2P2S5⋅0.5py after overnight crystallization (reaction pictured). This reactant, together with Li2S, was then used to synthesize Li3PS4 in an acetonitrile solution.
Using this approach, the synthesis time of Li3PS4 was significantly reduced—from days to hours. This is due to the increased reactivity of the P2S5 complexes. This work has implications for a better understanding of electrolyte synthesis and for developing improved processes.
- Pyridine Complexes as Tailored Precursors for Rapid Synthesis of Thiophosphate Superionic Conductors,
Michael Ghidiu, Roman Schlem, Wolfgang G. Zeier,
Batteries Supercaps 2021.
https://doi.org/10.1002/batt.202000317