To overcome the sluggish kinetics and high overpotential, electrocatalysts for oxygen reduction reaction (ORR) are necessary for both fuel cells and metal–air batteries. Considering the scarce resources and high cost of Pt-based catalysts, non-noble transition-metal macrocyclic complexes are regarded as a potential alternative. These catalysts have the advantages of resource abundance, cost-effectiveness, atom economy, and structure designability. However, the role of macrocyclic ligands in ORR on metal macrocyclic complexes is still not clear.
Jun Chen and colleagues, Nankai University, China, have investigated the role of aromaticity/antiaromaticity effect of macrocyclic ligands on the activity of transition metal macrocyclic complexes for electrocatalytic ORR by first-principles calculations. The team used three series of complexes with different aromatic or antiaromatic macrocycles, including TM norcorrole (TM = Mn, Fe, Co, Ni), TM porphycene, and TM porphyrin, as examples.
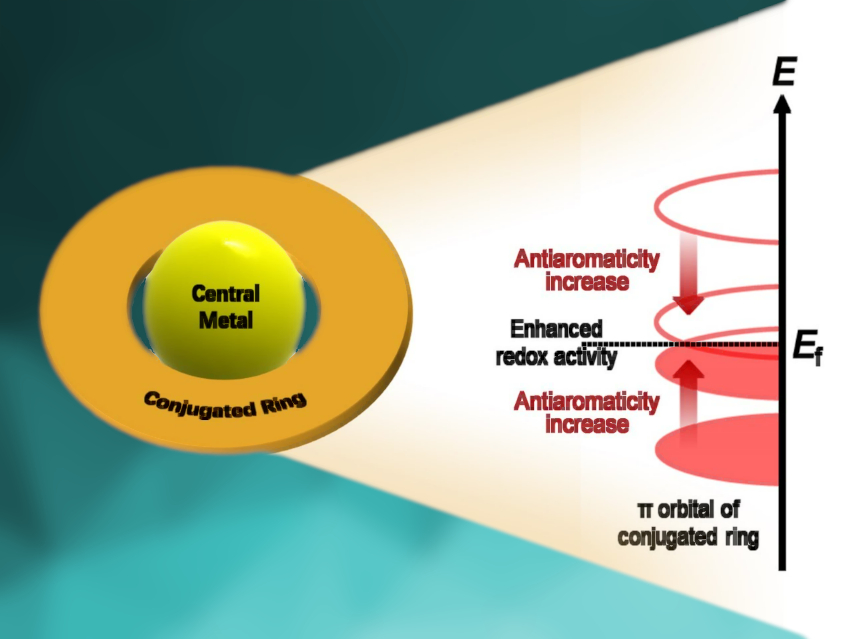
The different π-electronic structures of the macrocycles with different aromaticities affect the active d-electrons of the TM centers via d–π conjugation. The involvement of macrocycles in the oxygen electroreduction process enhances the adsorption of intermediates to the TM macrocycles to different degrees. TMPc with the weak aromatic Pc shows stronger adsorption for O species than TMP, which has the strong aromatic P. In contrast, TMNc with the antiaromatic Nc shows the strongest adsorption. Therefore, different TM centers require matching macrocycles to achieve a moderate adsorption strength for higher ORR activity.
For example, Mn and Fe centers with initially strong activity can show higher ORR activity when coordinated with aromatic P. Among all TML electrocatalysts (TM = Mn, Fe, Co, Ni; L = Nc, Pc, P), CoPc has the highest ORR activity with an overpotential of 0.29 V due to its appropriate adsorption strength. The researchers expect that this work will inspire new ideas for the design of TM macrocyclic complex catalysts.
- Aromaticity/Antiaromaticity Effect on Activity of Transition Metal Macrocyclic Complexes towards Electrocatalytic Oxygen Reduction,
Youxuan Ni, Yong Lu, Kai Zhang, Jun Chen,
ChemSusChem 2021.
https://doi.org/10.1002/cssc.202100182