Johann Josef Loschmidt was born on March 15, 1821, in Putschirn near Karlsbad, Austria (now Karlovy Vary, Czech Republic) as a child of poor farmers. The priest Adalbert Czech persuaded his parents to send the boy to high school in a monastery in Schlackenwerth (today Ostrov, Czech Republic) and then to advanced high-school classes in Prague, today’s Czech Republic, from 1837 on. Loschmidt first studied philosophy and mathematics at Charles University, Prague. In 1841, he moved to Vienna, Austria, where he studied physics and chemistry. He graduated in 1846.
Loschmidt failed to find an academic post at first and worked in a steel factory, founded an (ultimately unsuccessful) potassium nitrate factory, and then worked as a teacher before joining the University of Vienna in 1868 as Assistant Professor. He was promoted to Professor of Physical Chemistry in 1872 and served in this position until his retirement in 1891. One of his students there was Ludwig Boltzmann. Josef Loschmidt died on July 8, 1895, in Vienna.
Josef Loschmidt worked, e.g., on thermodynamics, optics, electrodynamics, and crystal forms. He is best known for two ground-breaking contributions to chemistry: developing an early chemical structure notation that is fairly similar to modern representations [1] and estimating the size of the molecules that make up the air [2,3].
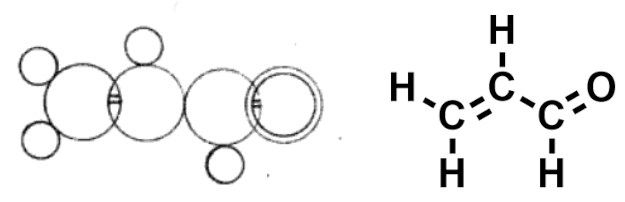
In his booklet Chemische Studien (chemical studies), published in 1861 [1], Loschmidt proposed two-dimensional representations for the structures of over 300 molecules that look fairly similar to the structural formulas used by chemists today (see Fig. 1). Loschmidt’s formulas show the spatial arrangement of atoms as circles and include multiple bonds as parallel lines—just like multiple bonds in modern notation.
 |
|
Figure 1. Structure of acrolein in Loschmidt’s notation ([1], left) and modern representation (right). |
In 1865, Loschmidt was the first to estimate the size of the molecules in air based on gas kinetics [2,3]. His work allowed him to determine how many molecules are present in a given volume of an ideal gas, a quantity known today as the Loschmidt constant n0 in his honor. Its value is ca. 2.69·1025 molecules/m3 at 0 °C and 1 atm.
Josef Loschmidt is the answer to Guess the Chemist (111).
References
- [1] Chemische Studien,
J. Loschmidt,
Carl Gerold’s Sohn, Vienna, 1861. - [2] Zur Grösse der Luftmoleküle (in German)
J. Loschmidt,
Sitzungsberichte d. Kaiserlichen Akademie d. Wissenschaften Wien 1865, 52, 395–413. - [3] Loschmidt and the Discovery of the Small,
W. W. Porterfield, W, Kruse,
J. Chem. Educ. 1995, 72, 870.
https://doi.org/10.1021/ed072p870.1
(English translation of Loschmidt’s “On the Size of Air Molecules”)
Sources
- Loschmidt, Johann Joseph (in German),
R. Knott,
Allgemeine Deutsche Biographie 1906, 52, 82–84. - Loschmidt, Johann Joseph (in German),
C. Priesner,
Neue Deutsche Biographie 1987, 15, 195. - Joseph Loschmidt: Structural formulae, 1861,
H. S. Rzepa,
www.ch.ic.ac.uk/rzepa/loschmidt, 2005.


