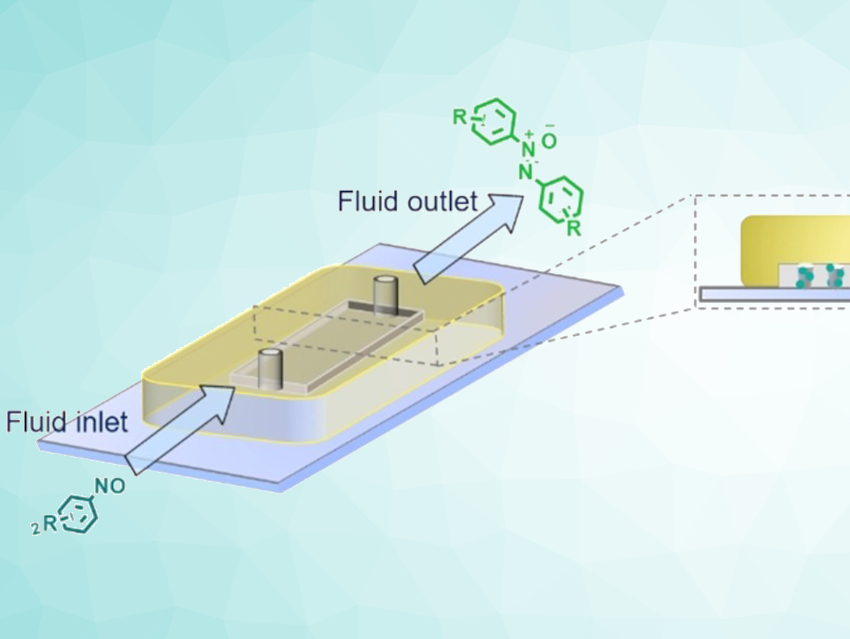
Flow reactors are useful tools for continuous organic reactions with immobilized catalysts. Microfluidic reactors (MFRs) with small dimensions have advantages such as excellent heat exchange and a laminar flow. However, the fixation of catalysts within MFRs is quite challenging. To overcome this problem and to improve the catalytic activity inside the flow reactor, catalysts can be integrated within crosslinked polymer networks. Using relatively low crosslinker contents enables reactions inside of swollen, sponge-like, polymer networks. Compared with solid particles, this increases the amount of immobilized and accessible catalyst.
Dirk Kuckling, Paderborn University, Germany, and colleagues have developed an approach for the formation of a wide range of azoxybenzenes via the reductive dimerization of nitrosobenzenes using a microfluidic reactor with gel-bound proline organocatalysts (pictured). The team converted mono‐2‐(methacryloyloxy)ethyl succinate into the corresponding acid chloride using thionylchloride. This acid chloride was then treated with trans‐4‐hydroxy‐L‐proline in trifluoroacetic acid (TFA) to generate a proline ester derivative. Finally, the ester was immobilized via copolymerization with methyl methacrylate (MMA) and ethylene glycol dimethacrylate (EGDMA) to form a gel network.
The resulting gel was placed in an MFR, which was then used for the reductive dimerization of nitrosobenzenes. Azoxybenzenes were formed within minutes at mild conditions in good to almost quantitative yields. According to the researchers, this work could easily be extended to other reactions under continuous flow conditions, e.g., aldol reactions.
- Continuous flow synthesis of azoxybenzenes by reductive dimerization of nitrosobenzenes with gel-bound catalysts,
Carsten J. Schmiegel, Patrik Berg, Franziska Obst, Roland Schoch, Dietmar Appelhans, Dirk Kuckling,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100006