The enzyme AsqJ is a dioxygenase found in the fungus Aspergillus nidulans. It catalyzes the key step in the biosynthesis of 4′-methoxyviridicatin antibiotics. Expanding the natural substrate scope of this enzyme could, therefore, help with the development of new antibiotics.
Tobias A. M. Gulder, Technical University of Dresden, Germany, K. N. Houk, University of California, Los Angeles, USA, and colleagues have discovered a dual function of AsqJ. The team used a streamlined solid-phase peptide synthesis to prepare a diverse set of benzo[1,4]diazepine-2,5-dione substrates (pictured below). The resulting compound library was then used to study the behavior of AsqJ and its potential catalytic promiscuity.
The team found that, depending on just a single substituent within complex substrate structures, AsqJ either produces quinolone or quinazolinone alkaloid scaffolds (pictured below). The new product class is formed following an entirely new enzymatic mechanism, which the researchers validated by in-depth functional and computational studies.
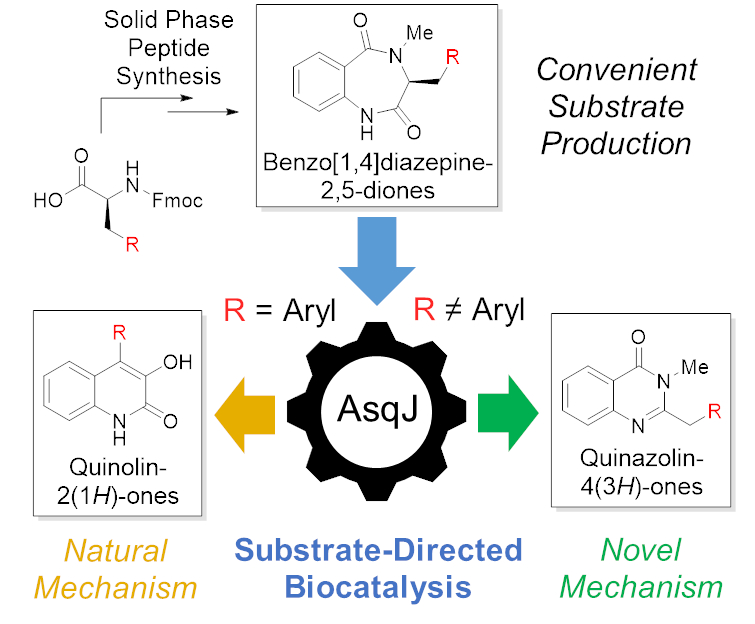
This work unlocks a second, “unnatural” function of AsqJ. The substrate-directed product selectivity could be used to enable the efficient synthesis of different, biomedically relevant alkaloid scaffolds.
- Fungal Dioxygenase AsqJ Is Promiscuous and Bimodal: Substrate‐Directed Formation of Quinolones versus Quinazolinones,
Manuel Einsiedler, Cooper S. Jamieson, Mark A. Maskeri, Kendall N. Houk, Tobias A. M. Gulder,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202017086