Chlorine-functionalized organic molecules are versatile starting materials for organic syntheses. The ability to exchange a specific hydrogen atom on a molecule with chlorine would be useful. However, in practice it is challenging to carry out selective sp3 C–H chlorinations.
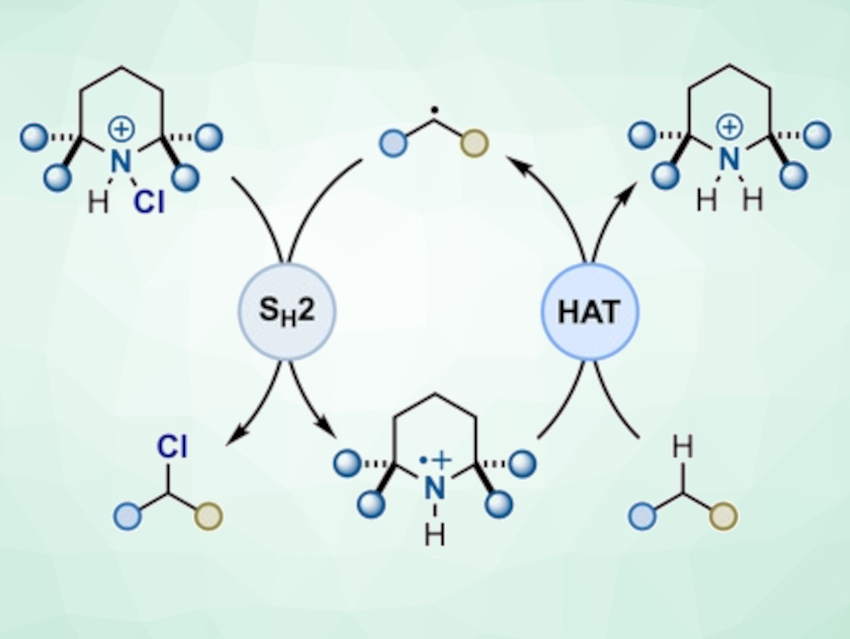
Alessandro Ruffoni, Daniele Leonori, University of Manchester, UK, Massimo Bietti, Tor Vergata University of Rome, Italy, and colleagues have developed a photochemical method for C–H chlorination (pictured). The researchers chlorinated a simple amine, such as 2,2,6,6‐tetramethylpiperidine, with N‐chlorosuccinimide (NCS) to give an N-chloroamine. A radical reaction is initiated with blue light from a light-emitting diode (LED), and an aminium radical is generated from the amine by a radical chain propagating process. The radical abstracts a H atom from an organic molecule to generate the corresponding carbon radical, which can then be chlorinated. In this process, the N-chloroamine serves as an aminium radical precursor, a hydrogen-abstracting species, and a chlorine-atom donor.
According to the researchers, this general approach targets specific positions by harnessing both steric and polar effects. This provides high selectivities for sp3 C–H chlorinations.
- Practical and Selective sp3 C−H Bond Chlorination via Aminium Radicals,
Alastair J. McMillan, Martyna Sieńkowska, Piero Di Lorenzo, Gemma K. Gransbury, Nicholas F. Chilton, Michela Salamone, Alessandro Ruffoni, Massimo Bietti, Daniele Leonori,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202100030