Highly ordered arrays of organic molecules on solid surfaces are interesting research targets that can be studied using scanning tunneling microscopy (STM) or high-resolution atomic force microscopy (AFM). For STM investigations, highly oriented pyrolytic graphite (HOPG) is often used as a substrate. However, assembling three-dimensional molecules on graphite at the solid/liquid interface can be challenging.
Stefan-S. Jester, Sigurd Höger, University of Bonn, Germany, and colleagues have synthesized bicyclophanes and visualized self-assembled monolayers of these compounds using STN with submolecular resolution. Bicyclophanes are molecular frameworks in which phenylene units are connected to form large fused rings that are divided into two smaller sub-rings. The team used a twofold Fischer‐Zimmermann condensation of appropriate pyrylium salts with arylene dicarboxylic acid salts to obtain tetrabromo aromatics. These intermediates were converted to highly strained bicyclophanes with different sizes and different intraannular bridges via intramolecular Yamamoto couplings.
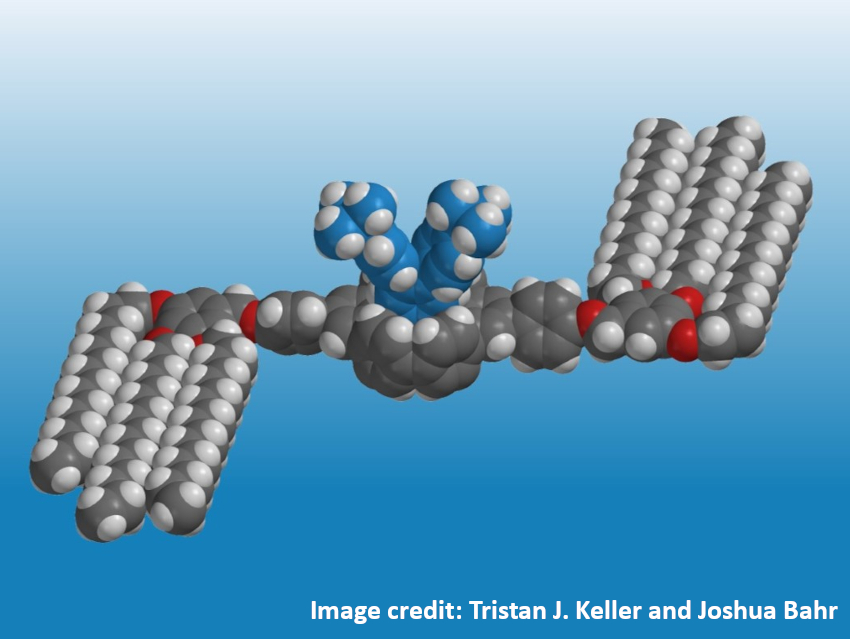
The team found that after adsorption, the central units can point towards the volume phase. In the pictured example with long, adsorbed alkoxy side chains, the rotational degrees of freedom of the central unit are restricted so that two covalently attached units (pictured in blue) point out of the molecular plane. Therefore, these molecules are attractive platforms for addressing the volume phase above a graphite surface. In the future, this approach could allow researchers to covalently attach functional units to similarly robust molecular pillars, performing highly specific tasks at the interface.
- Highly Strained Nanoscale Bicyclophane Monolayers Entering the Third Dimension: A Combined Synthetic and Scanning Tunneling Microscopy Investigation,
Sebastian Henzel, Steven Becker, Daniel Hennen, Tristan J. Keller, Joshua Bahr, Stefan‐S. Jester, Sigurd Höger,
ChemPlusChem 2021.
https://doi.org/10.1002/cplu.202000711