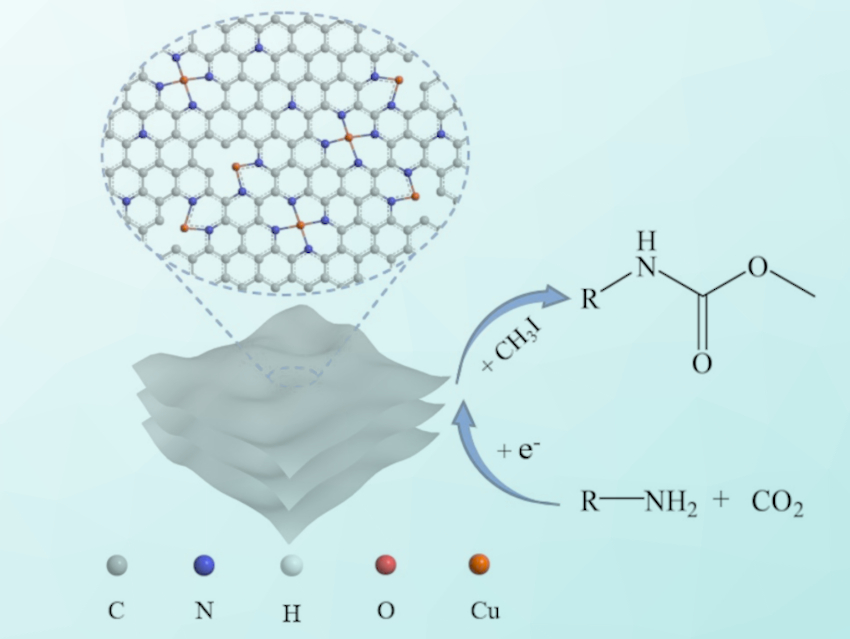
Using electrocatalysis, carbamates (RO–C(=O)–NR’R”) can be synthesized from CO2 and amines under mild conditions. Such reactions do not require added dehydrating agents, bases, or homogeneous catalysts. However, the method has a limited conversion performance for certain amine substrates. To improve the efficiency in these cases, better catalysts are needed as electrode materials. Single-atom catalysts with atomically dispersed metals are promising candidates for this.
Jia-Xing Lu, Huan Wang, East China Normal University, China, and colleagues have prepared a material featuring atomically dispersed Cu species on N-doped carbon nanosheets (Cu-N-C) as electrocatalysts for the synthesis of carbamates. The team used a Cu‐containing MOF—made using 1,3,5‐benzenetricarboxylic acid, Cu(CH3COO)2 ⋅ H2O, and L‐glutamic acid—as a precursor, together with dicyandiamide as a nitrogen source. These reactants were calcined under an N2 atmosphere at 800 °C and then etched using hydrochloric acid in air to obtain the desired Cu-N-C catalyst.
Cu-N-C exhibits much better catalytic performance than the traditional Cu bulk for the electrocatalytic synthesis of N-phenylcarbamate, and it reaches 71 % yield along with good stability over 10 cycles. The catalyst can be used to smoothly convert various amines into the corresponding carbamates. According to the researchers, the atomically dispersed copper provides abundant active sites, and the pyridine-type N atoms assist by stabilizing the intermediate during the reaction.
- Atomically Dispersed Copper on N‐Doped Carbon Nanosheets for Electrocatalytic Synthesis of Carbamates from CO2 as a C1 Source,
Shi‐Ming Li, Yi Shi, Jing‐Jie Zhang, Ying Wang, Huan Wang, Jia‐Xing Lu,
ChemSusChem 2021.
https://doi.org/10.1002/cssc.202100342