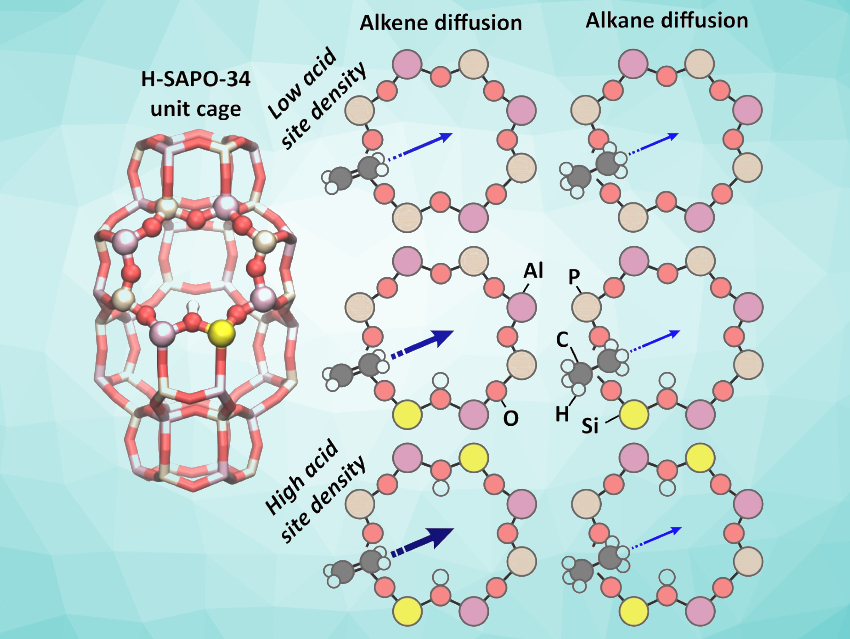
Zeolite catalysts are widely used in industrial processes. The diffusion of hydrocarbons is important for many zeolite‐catalyzed processes. The transport of small alkenes in the confined zeolite pores can become hindered, resulting in a significant impact on the product selectivity and separation. The distribution of acid sites in the catalyst is an important parameter for many catalytic processes, but the precise influence of the acid-site density and distribution on the transport properties in zeolites is not well understood.
Veronique Van Speybroeck, Ghent University, Zwijnaarde, Belgium, and colleagues have analyzed the influence of the acid-site density on the diffusion of small alkenes and alkanes in the zeolite H-SAPO-34 (pictured). The team used molecular dynamics (MD) simulations to probe the “hopping” of individual molecules between neighboring catalyst cages. Pulsed-field gradient NMR spectroscopy was used to measure diffusivities within large model crystallites, and so-called Temporal Analysis of Products (TAP) pulse‐response experiments allowed the researchers to study the adsorption and pore entry in small crystallites.
The team found that Brønsted-acid sites promote the diffusion of ethene and propene through the H-SAPO-34 cages. They attribute this effect to stabilizing π–H interactions with acid protons. Alkane diffusion is insensitive to the acid-site density. According to the researchers, the work could be helpful for the synthesis of zeolites with tailored acid-site distributions for catalysis and alkene–alkane separation applications.
- Experimental and Theoretical Evidence for the Promotional Effect of Acid Sites on the Diffusion of Alkenes through Small-Pore Zeolites,
Pieter Cnudde, Evgeniy A. Redekop, Weili Dai, Natale G. Porcaro, Michel Waroquier, Silvia Bordiga, Michael Hunger, Landong Li, Unni Olsbye, Veronique Van Speybroeck,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202017025