“Now Available with a Negative Charge”
The incorporation of boron into polycyclic aromatic hydrocarbon (PAH) systems leads to interesting chromophoric and fluorescing materials for optoelectronics, including organic light-emitting diodes (OLEDS) and field-effect transistors, as well as polymer-based sensors. David J. D. Wilson, Latrobe University, Melbourne, Australia, Robert J. Gilliard Jr., University of Virginia, Charlottesville, USA, and colleagues have introduced a new anionic organoborane compound. The synthesis of the borafluorene succeeded through the use of carbenes.
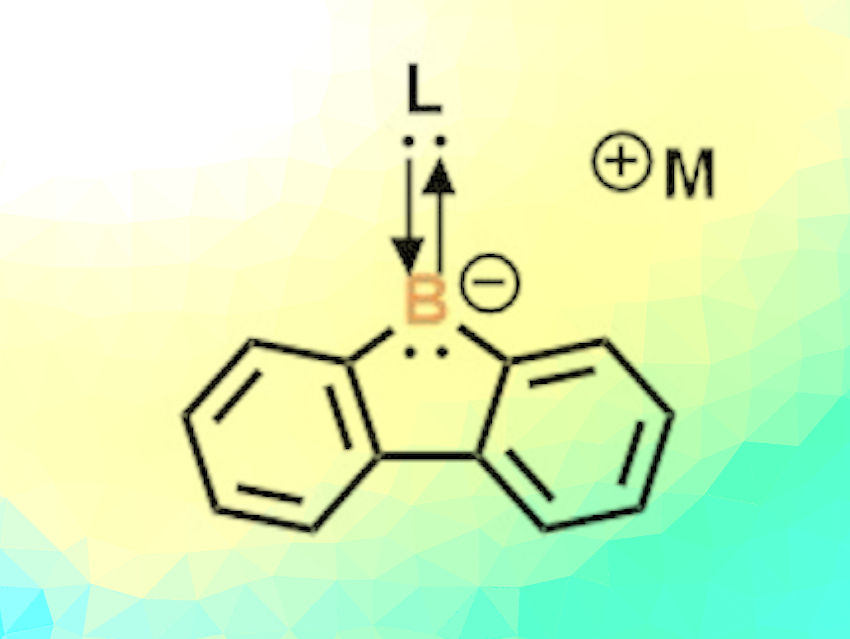
Borafluorene is a particularly interesting boron-containing building block. It is a system of three carbon rings: two six-membered rings and one central five-membered ring incorporating a boron atom. While neutral, radical, and cationic borafluorene compounds are quite easy to produce, there have been few examples of anionic borafluorene compounds to date. A better understanding of their chemistry is important for advances in redox-dependent applications and could lead to new materials with unique bonding or optical properties. However, the relatively high reactivity of borafluorene anions makes their synthesis challenging. The researchers succeeded in the isolation and structural characterization of these elusive anions.
Carbene-Stabilized Anions
The starting point for the team’s synthesis is 9-bromo-9-borafluorene, which has a bromine atom attached to its boron atom. This is treated with a very strong reducing agent (potassium graphite, sodium naphthalenide, or lithium naphthalenide) in the presence of carbenes. The anionic borafluorenes formed in the reduction are stabilized by the carbenes (pictured).
As the team demonstrated, the carbene–borafluorene anions can also be used as chemical building blocks. This makes it possible to produce new compounds that are not otherwise accessible with previously known starting materials. For example, compounds with bonds between boron and gold, selenium, or germanium were generated. A reaction with a diketone led to a ring closure and bonding of the boron atom to both ketone oxygens, forming a spirocyclic boron compound.
- Stabilization of the Elusive 9‐Carbene‐9‐Borafluorene Monoanion,
Kelsie E. Wentz, Andrew Molino, Sarah L. Weisflog, Aishvaryadeep Kaur, Diane A. Dickie, David J. D. Wilson, Robert J. Gilliard,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202103628