The chlorination of arenes via electrophilic aromatic substitution (SEAr) reactions is an important example of C–H functionalization. This strategy is particularly useful in drug design, because the introduction of a chlorine substituent to a medicinally active compound can be used to tune its pharmacokinetic properties. Iodobenzene dichloride (PhICl2) is an easy-to-handle solid that acts as a chlorinating agent. It could be a promising alternative to chlorine gas in SEAr reactions. However, it is a weaker oxidizing agent than Cl2.
Tiffany Poynder, Sevan Houston, and Jason Dutton, La Trobe University, Melbourne, Australia, have found that the activity of PhICl2 can be enhanced using readily accessible [Ag]2[B12Cl12] as a catalyst. This silver salt has a weakly coordinating carborane anion. The team chlorinated a variety of arenes at room temperature using PhICl2 as a chlorinating agent, [Ag]2[B12Cl12] as a catalyst, and CHCl3 as the solvent. The method was also extended to the dichlorination of alkenes and alkynes. The researchers propose a “iodonium”-type mechanism (pictured below), in which Ag+ abstracts a chloride ion from PhICl2, resulting in an active [PhICl]+ species, which might be stabilized by the weakly coordinating [B12Cl12]2– anion.
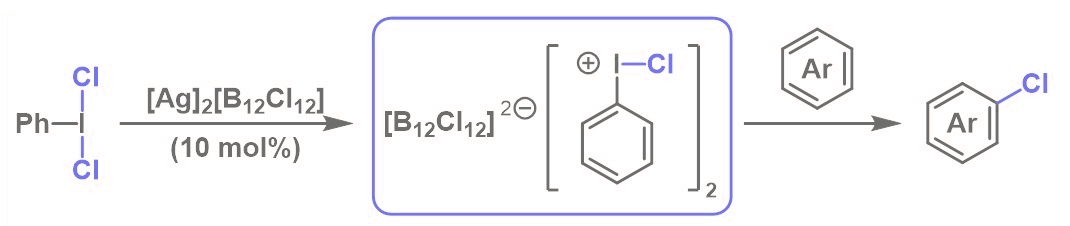
[Ag]2[B12Cl12] outperformed several commercially available Ag(I) salts. [Ag]2[B12Cl12] and related carborane reagents are typically only used by the inorganic chemistry community, but the researchers hope that this work encourages others to further investigate its application in organic synthesis.
- [Ag]2[B12Cl12] as a Catalyst in PhICl2 Mediated Chlorination,
Tiffany B. Poynder, Sevan David Houston, Jason L. Dutton,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100447
![[Ag]2[B12Cl12] as a Catalyst in Chlorination Reactions](https://www.chemistryviews.org/wp-content/uploads/legacy/common/images/thumbnails/source/17955c969f9.jpg)
![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)