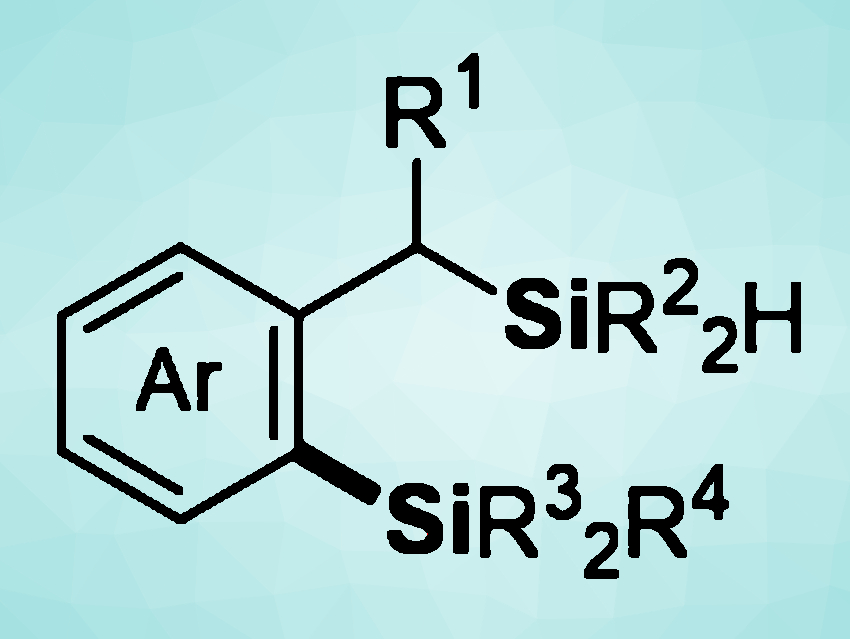
Organosilanes can play a range of important roles in synthetic chemistry, medicinal chemistry, agrochemistry, or materials science. Effective methods for the preparation of these compounds are, thus, interesting research targets.
Transition-metal catalyzed C−H silylations, for example, can be used to prepare silicon-containing molecules with high efficiency and atom-economy. However, the direct intermolecular silylation of C−H bonds using hydrosilanes as the silylation reagents usually requires harsh conditions and a large excess of arene substrates. Directing groups can also be necessary, which causes a need for additional reaction steps. However, if a hydrosilyl group could serve as a directing group for selective C−H activation and enable an intermolecular dehydrogenative C−H/Si−H cross-coupling with another hydrosilane partner, bis(silane) compounds (example pictured above) could be obtained.
Chuan He, Southern University of Science and Technology, Shenzhen, China, and colleagues have developed such an intermolecular dehydrogenative C−H/Si−H cross-coupling for the construction of bis(silane) compounds, catalyzed by an iridium complex (pictured below). The team used a wide range of aryldimethylsilane silylation agents, which were reacted with substates such as benzhydryldimethylsilane in the presence of [Ir(cod)OMe]2 (cod = 1,5-cyclooctadiene) and an amine-oxazoline ligand as a catalyst system. The reactions were performed at 50 °C.
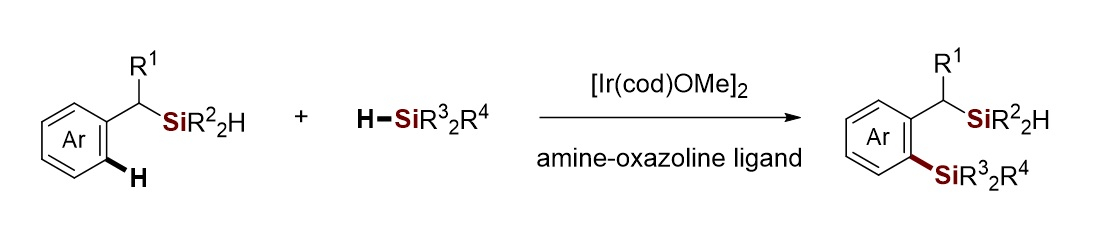
With this approach, the researchers obtained a variety of new functionalized arylbenzyl bis(silane) compounds under mild conditions. The reactions provided moderate to excellent yields and good chemo- and regioselectivity. The arylbenzyl bis(silanes) can be further functionalized and serve as versatile building blocks in synthetic organic chemistry.
- Intermolecular Dehydrogenative C−H/Si−H Cross‐Coupling for the Synthesis of Arylbenzyl Bis(silanes),
Lijun You, Wei Yuan, Chuan He,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100474