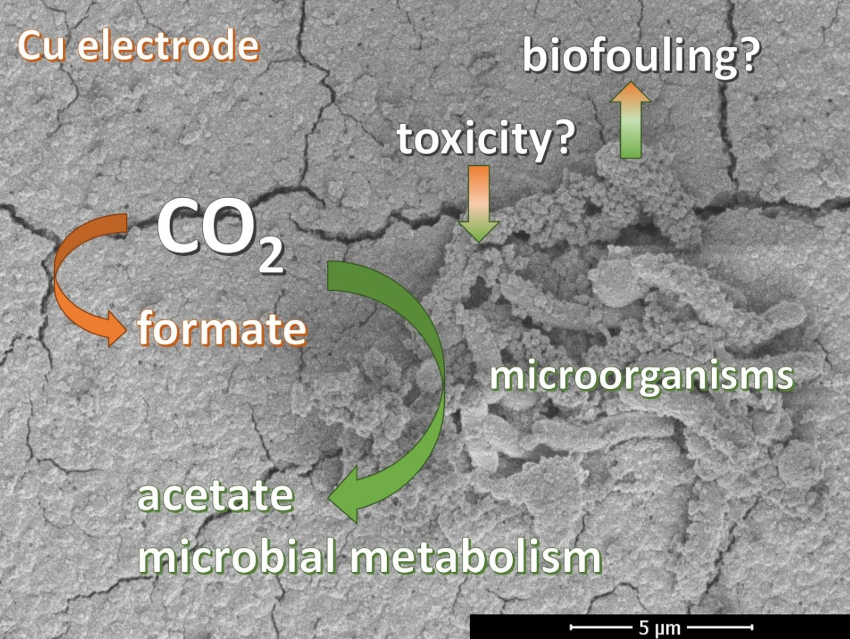
The electrocatalytic conversion of CO2 can be used to store renewable energy in the form of fuels or other chemicals, while using a greenhouse gas as the feedstock. Both metals and microorganisms can catalyze this reaction. Metals exhibit high reaction rates, but often deactivate rapidly and produce short carbon chains. In microbial electrosynthesis, the microorganisms have a lag phase, but are self-replicating, can operate for months, and produce long carbon chains.
Thus, metals and microorganisms could complement each other if combined. Metals can produce intermediates at high rates, which can be further converted by the microorganisms into longer-chain final products. The consumption of intermediates can kinetically improve the metal-catalyzed reaction. However, the combination of these two components can result in biofouling of the metal catalyst or metal toxicity to the microorganisms.
J. Harry Bitter, David P. B. T. B. Strik, Wageningen University & Research, The Netherlands, and colleagues have investigated the combination of a copper electrocatalyst and microorganisms in one reactor for CO2 conversion. To separate the effects of the components, the team performed four types of electrochemical experiments: abiotic copper electrosynthesis without microbes, microbial electrosynthesis with copper electrodes, copper electrosynthesis in the presence of deactivated microorganisms, and microbial electrosynthesis with copper electrodes in reactors with pre-grown biocatalyst on a graphite felt electrode. The team used copper oxide (CuOx) or copper-foil electrodes, together with a mixture of microorganisms from a mature microbial community in a “parent” bio-reactor.
The team found that both catalysts appeared active upon combination in one reactor. Despite its antimicrobial properties, copper allowed the formation of a biofilm on the surface of the electrode. Formate was produced from CO2 promoted by the copper electrocatalyst. The microorganisms consumed CO2 and formate, producing acetate as a final product. Thus, Cu is a promising catalyst to combine with microorganisms.
- Catalytic Cooperation between a Copper Oxide Electrocatalyst and a Microbial Community for Microbial Electrosynthesis,
Konstantina-Roxani Chatzipanagiotou, Virangni Soekhoe, Ludovic Jourdin, Cees J. N. Buisman, J. Harry Bitter, David P. B. T. B. Strik,
ChemPlusChem 2021.
https://doi.org/10.1002/cplu.202100119