Polymers that translate mechanical stresses into optical signals are useful for many applications, e.g., for damage detection in load-bearing structures. Mechanically responsive units (mechanophores) that undergo covalent bond breaks in response to mechanical force are often used to create materials with such properties. However, the response of such mechanophores is usually irreversible and unspecific.
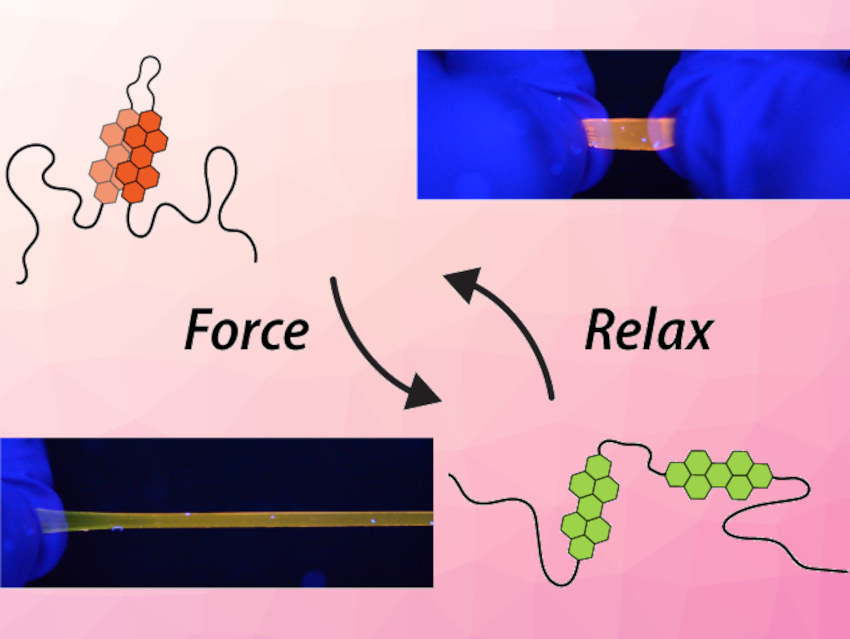
Christoph Weder, University of Fribourg, Switzerland, and colleagues have created a supramolecular mechanophore that works differently. They connected two fluorescent perylene dyes with a spacer and incorporated this motif into the center of poly(methylacrylate) chains (schematically pictured above).
The team started from commercially available perylene-3,4,9,10-tetracarboxylic dianhydride, which was converted to a perylene diimide dialcohol by imide formation with tetraethylene glycol monoamine. This was followed by esterifications with α-bromoisobutyryl bromide and with adipic acid to give an initiator in which two perylene diimide units are separated by a spacer. This initiator was used to polymerize methyl acrylate by atom transfer radical polymerization (ATRP) to give the desired polymer that features a perylene-diimide loop.
In the stress-free state, the perylenes dimerize (pictured in orange), the mechanophores fold into loops, and the interactions among the luminescent dyes lead to an orange emission. When the polymer is stretched, the loops unfold, the perylenes separate (pictured in green), and their fluorescence changes to green. This change is instant, reversible, clearly discernible, and correlated to the applied strain. The motif could also be incorporated into other polymers.
- Folded Perylene Diimide Loops as Mechanoresponsive Motifs,
Hanna Traeger, Yoshimitsu Sagara, Derek J. Kiebala, Stephen Schrettl, Christoph Weder,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202105219