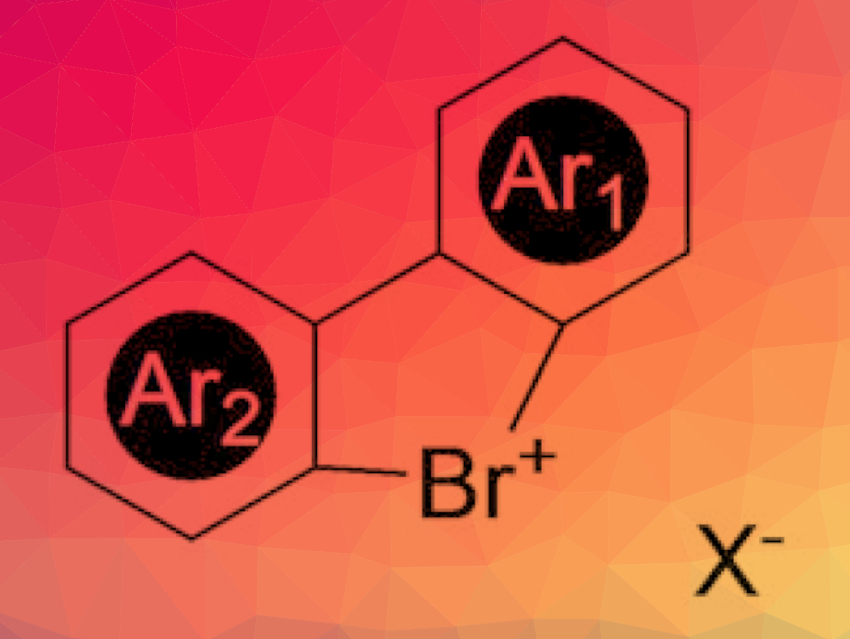
Diaryl λ3-bromanes are a rare and generally unresearched class of compounds. Joanna Wencel-Delord, Université de Strasbourg/Université de Haute Alsace, Strasbourg, France, and colleagues have developed a mild and efficient protocol that delivers a broad palette of these interesting building blocks for organic synthesis. These compounds have a higher reactivity and different selectivity than the corresponding iodane compounds.
Hypervalent Bromine Compounds
Halogen atoms usually have an oxidation level of −1 and only one bonding partner. However, iodine and bromine can also adopt oxidation levels of +3 or +5 and participate in multiple bonds. Such compounds are known as hypervalent iodines and bromines. Hypervalent iodines, in particular, play an important role in organic synthesis. For example, diaryl λ3-iodanes (positively charged iodine atoms bound to two aromatic six-membered carbon rings) are interesting building blocks for the synthesis of complex molecules, in particular, via metal-catalyzed reactions.
The corresponding hypervalent bromine compounds have received much less attention. One reason for this is that diaryl λ3-bromanes are more difficult to produce, usually by reactions requiring multiple steps under harsh reaction conditions or using extremely corrosive and toxic reagents and giving poor yields.
General, Safe, and High-Yielding Synthesis
The team has developed a versatile and high-yielding synthetic strategy that provides access to a broad palette of stable, non-air-sensitive, cyclic diaryl λ3-bromanes (pictured). The key step is a ring closure that forms a system consisting of two aromatic six-membered rings that are connected through a five-membered ring. The “tip” of the five-membered ring is the hypervalent, positively charged bromine atom. The team produced a series of different variants with different side groups and counterions.
The cyclic bromanes proved to be interesting synthetic building blocks. In the presence of a weak base, the five-membered ring opens, allowing for C–O and C–N couplings—even without the need for a metal catalyst. Interestingly, the C–O and C–N bonds are formed on the unexpected meta position. In addition, this reactivity cannot be reached while using the corresponding diaryl iodanes, thus, clearly illustrating the complementarity of these hypervalent species.
The cause of the different selectivity is a different reaction mechanism. In the case of the iodanes, and in the presence of a metal catalyst, attack of the reaction partner leads to splitting of the carbon–iodine bond, which is in the ortho position, followed by coupling of this ortho carbon atom when the ring opens. In the case of the bromanes, in contrast, the first step of the reaction is the deprotonation of the aromatic ring and cleavage of one of the carbon–bromine bonds and formation of an aryne ring, which contains a triple bond within the aromatic ring between the ortho and meta carbon atoms. When the coupling reagent in the next step attacks this triple bond, this preferentially occurs at the meta carbon due to the combination of electronic and steric effects.
- Cyclic Diaryl λ3‐Bromanes as Original Aryne Precursors,
Matteo Lanzi, Quentin Dherbassy, Joanna Wencel‐Delord,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202103625