Highly dispersed platinum catalysts provide new possibilities for industrial processes, such as the flameless combustion of methane, propane, or carbon monoxide, which has fewer emissions and is more resource-efficient and consistent than conventional combustion. Pedro Serna, ExxonMobil Research and Engineering Co., Annandale, NJ, USA, Manuel Moliner, Avelino Corma, Universitat Politècnica de València—Consejo Superior de Investigaciones Cientificas (UPV-CSIC), Valencia, Spain, and colleagues have investigated which platinum species are active in high-temperature oxidations and what changes they can undergo in the course of the process—important prerequisites for the optimization of catalysts.
Individual Atom or Metal Cluster?
Individual metal atoms and clusters consisting of only a few metal atoms have interesting catalytic properties determined by the exact nature of the active metal species. Usually, these are highly dispersed and deposited on a support such as zeolite, which is a porous silicate framework structure that also plays a role in the characteristics of a catalyst. Even the smallest change in the active centers can drastically reduce the efficiency of a catalyst. For example, noble metals like platinum tend to become permanently deactivated through sintering under harsh conditions.
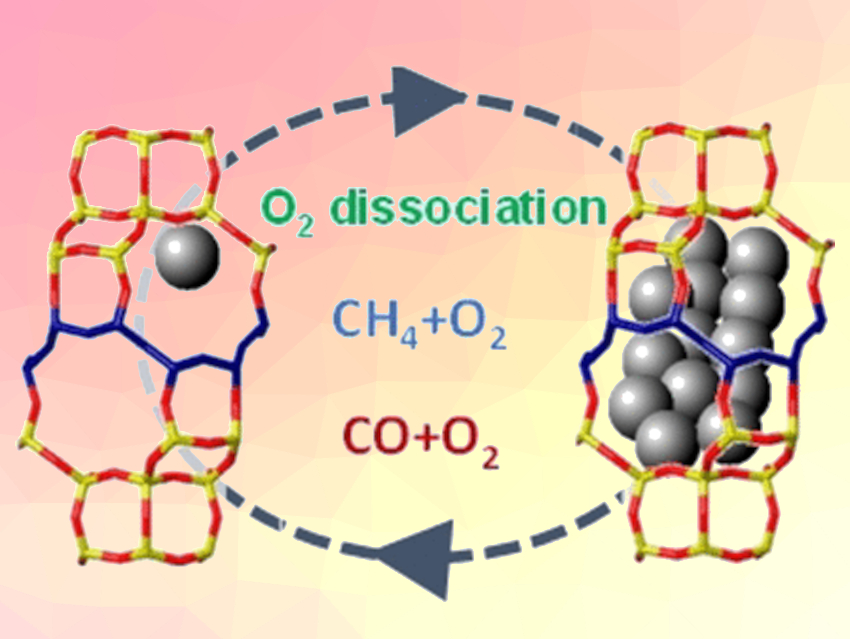
Which specific platinum species play a role in high-temperature oxidations is hard to determine, however, because a significant number of such species cannot readily be obtained without the involvement of their support in the catalysis. The team investigated the behavior of individual platinum atoms and small platinum clusters on special CHA zeolites, which are non-reducible supports that can stabilize these species very well.
O2 Dissociation and Oxidation Catalysis
The researchers’ first experiment was an investigation of splitting O2 using two different types of isotopically pure oxygen molecules, 16O2 and 18O2. The more active the catalyst, the more mixed 16O18O molecules are formed upon recombination of the dissociated atoms. It was shown that platinum clusters with sizes under 1 nm are significantly more active than individual atoms or larger clusters. However, at moderate temperatures (200 °C), the tiny clusters fall apart over time into individual platinum atoms and the catalytic activity for splitting oxygen ends.
In contrast, the team found that for the oxidation of alkanes, such as methane, at higher temperatures, the catalytic combustion was carried out by individual platinum atoms. These are formed in situ in the oxygen stream from the initial clusters, as was shown by X-ray absorption spectroscopy and by electron microscopy. The critical step in these oxidations is not the splitting of O2 but the breaking of C–H bonds, which is less sensitive to changes in the active-site structure.
Reactions Involving CO
For the oxidation of CO, the catalysis is dominated by platinum clusters. Individual platinum atoms cannot be stabilized in the CO stream, and thus, play no role. In comparison with supports made of aluminum oxide, the CHA zeolite provided higher activity and greater stability of the platinum clusters in the presence of CO.
The high stability of individual platinum atoms for methane combustion and of small platinum clusters for CO oxidation, which is retained after regeneration or treatment with hot steam, opens new possibilities for systems made of platinum and silicate zeolites as efficient and robust heterogeneous catalysts for a variety of high-temperature oxidation scenarios.
- Single‐Site vs. Cluster Catalysis in High Temperature Oxidations,
Pedro Serna, Aida Rodríguez‐Fernández, Sara Yacob, Christine Kliewer, Manuel Moliner, Avelino Corma,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202102339