Fuel cells can be used as energy sources with possible applications, e.g., in portable electronic devices or vehicles. The electrolyte is particularly important for the performance and safety of a fuel cell. Proton-conductive metal–organic frameworks (MOFs), for example, could be useful as solid-state electrolytes.
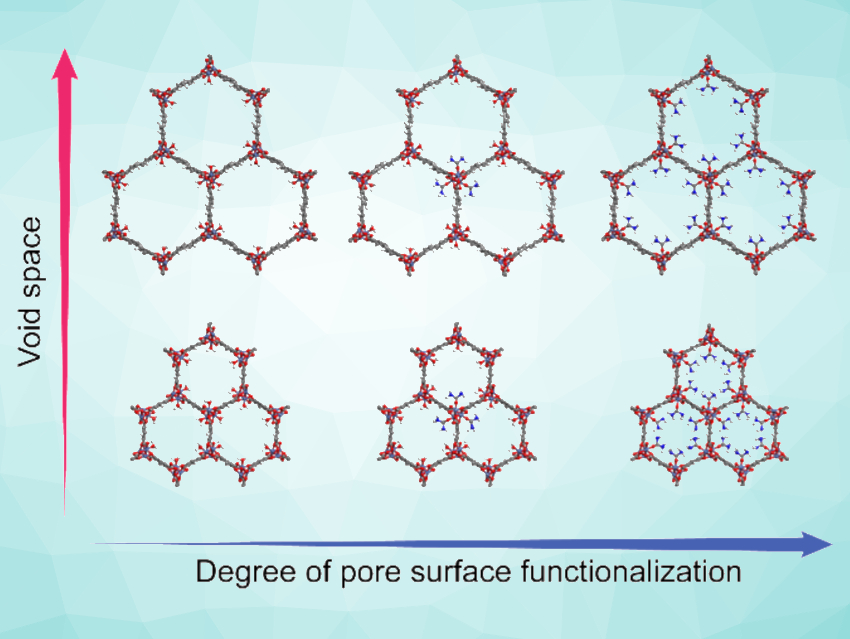
Jihan Kim, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Republic of Korea, Dae-Woon Lim, Yonsei University, Gangwon-do, Republic of Korea, Hiroshi Kitagawa, Kyoto University, Japan, and colleagues have studied the effects of the porosity and surface functionality of MOFs on their proton conductivity. The team synthesized a series of nickel-based MOF-74 variants of the types Ni2(dobdc) and Ni2(dobpdc) (dobdc = 2,5-dihydroxy-1,4-benzenedicarboxylate, dobpdc = 4,4′-dihydroxy-(1,1′-biphenyl)-3,3’-dicarboxylate), which have the same overall structure but different pore sizes (pictured bottom vs. top). In addition, the pore surface of some of the MOF samples was functionalized with urea, resulting in different degrees of urea coordination (pictured left to right).
The researchers measured the porosity and H2O adsorption properties of the MOFs using N2 gas and H2O vapor sorption, as well as the proton conductivity using impedance measurements. They found that the urea assists proton conduction by strengthening the hydrogen-bond network of the water confined in the MOF. Samples with larger pore diameters showed lower proton conductivities than samples with smaller pores and the same surface functionalization. This indicates that the confinement in smaller pores can be advantageous for obtaining high proton conductivities. The work could help to design efficient proton conductors.
- Void Space vs. Surface Functionalization for Proton Conduction in Metal–Organic Frameworks,
Marvin K Sarango-Ramírez, Junkil Park, Jihan Kim, Yukihiro Yoshida, Dae-Woon Lim, Hiroshi Kitagawa,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202106181