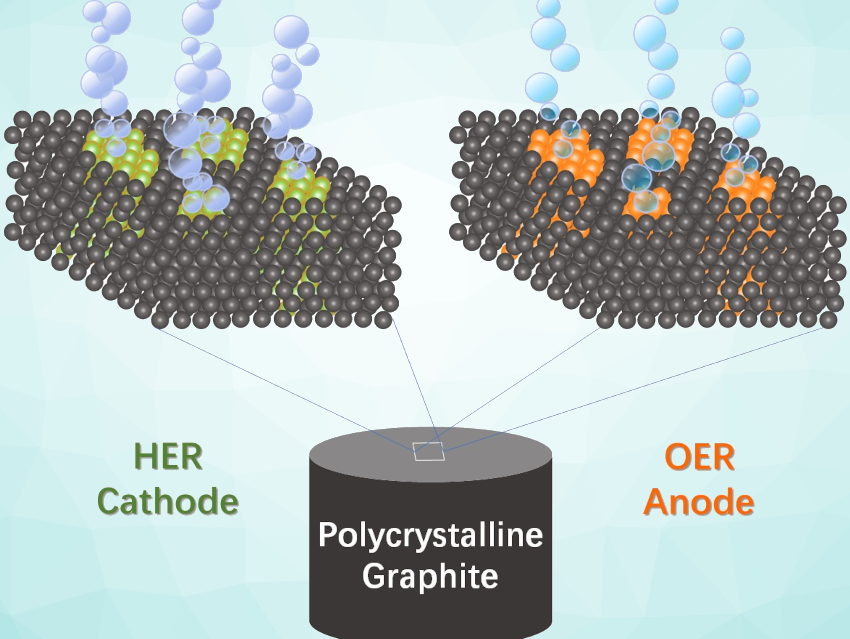
Water splitting driven by renewable energy could be used to provide hydrogen as a clean, sustainable fuel. Water splitting requires efficient catalysts for both the hydrogen and oxygen evolution reactions (HER and OER, respectively). Low-cost, efficient bifunctional catalysts for overall water splitting would be particularly useful.
Jian-Bo He, Hefei University of Technology, China, and colleagues have synthesized a bifunctional Ni-Fe-S electrocatalyst for both the hydrogen and oxygen evolution reactions in 1.0 M KOH. The team used intergranular defects or nanopores inside polycrystalline graphite as nanoreactors for the in-situ synthesis of the catalyst (pictured schematically). They used two precursor solutions, which can penetrate the intergranular nanopores in graphite to a depth of more than 3.5 mm and then form the catalytically active species. The researchers used solutions of thioacetamide (CH3CSNH2) as sulfur sources together with mixed precursor solutions of Ni(NO3)2 and Fe(NO3)3. These solutions were drop-cast onto graphite to obtain the desired nickel and iron (di-)sulfide (Ni-Fe-S) composite nanoparticles.
The graphite substrate provides a rigid conductive medium which lowers the electron transfer barrier between the catalyst particles and improves their stability. The resulting catalyst shows excellent catalytic activity and long-term stability at a high current density of 400 mA cm–2. The synthesis strategy based on using the nanopores inside a monolithic conductive substrate could provide a simple and efficient method for the preparation of electrocatalysts.
- High-performance bifunctional Ni-Fe-S catalyst in situ synthesized within graphite intergranular nanopores for overall water splitting,
Xiao-fan Yang, Jing Li, Xin-ming Yang, Chao-xiong Li, Fang Li, Bing Li, Jian-Bo He,
ChemSusChem 2021.
https://doi.org/10.1002/cssc.202100891