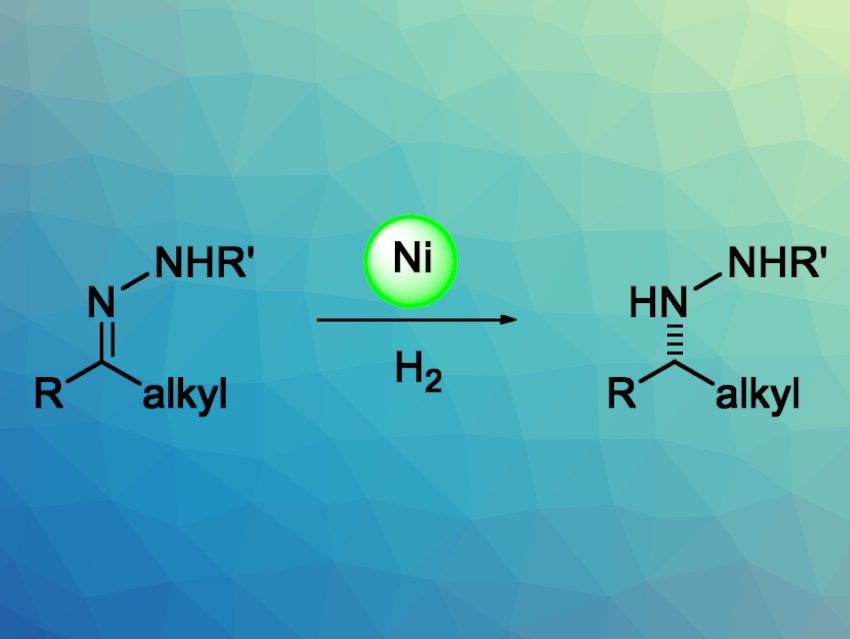
Chiral hydrazines are useful, e.g., in pharmaceutical and organic chemistry. The transition-metal-catalyzed asymmetric hydrogenation of hydrazones is an efficient method for the synthesis of chiral hydrazines. Noble-metal catalysts based on Rh, Ir, Pd, and Ru can be used for this reaction. The development of earth-abundant metal catalysts based on Mn, Fe, Co, Ni, or Cu for this type of asymmetric hydrogenation could reduce costs and improve sustainability. Although nickel catalysis has developed rapidly in recent years, there had been no examples of nickel-catalyzed asymmetric hydrogenations of hydrazones using H2 gas so far.
Wanbin Zhang, Shanghai Jiao Tong University and Zhengzhou University, both China, Zhenfeng Zhang, Shanghai Jiao Tong University, and colleagues have developed an efficient Ni-catalyzed asymmetric hydrogenation of hydrazones for the synthesis of chiral hydrazines (pictured). The team used Ni(OAc)2·4 H2O as a catalyst together with the chiral dialkyl phosphine ligand (R,R)-QuinoxP* and a mixture of 2,2,2-trifluoroethanol (TFE) and acetic acid as the solvent. The reactions were performed under 30 bar H2 at 50 °C.
The desired chiral hydrazines were obtained in excellent yields and moderate to excellent enantioselectivities from a wide range of aromatic and aliphatic hydrazones. The team was able to perform the reaction on a gram scale. The obtained hydrazines can be as used as intermediates for further transformations.
- Nickel-Catalyzed Asymmetric Hydrogenation of Hydrazones,
Bowen Li, Dan Liu, Yanhua Hu, Jianzhong Chen, Zhenfeng Zhang, Wanbin Zhang,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100642