Heavy metal pollution in water—in particular, industrial waste that contains chromium(VI) species—can be harmful to human health and the environment. The removal of hexavalent chromium from wastewater can be achieved via adsorption. However, many adsorbents have a low adsorption capability, leading to large amounts of harmful waste.
Reducing Cr(VI) to the less harmful Cr(III) is an alternative approach that could be performed in an environmentally friendly way with suitable photocatalysts. For example, bismuth oxyiodide (BiOI) shows photocatalytic activity for this reaction under visible light irradiation. However, it is hampered by high charge carrier recombination, which limits its activity.
Williams Kweku Darkwah, Hohai University, Nanjing, China, and University of Cape Coast, Ghana, and colleagues have investigated the effect of calcination temperature on the resulting phase and photocatalytic performance of bismuth oxyiodide. The team calcined BiOI at temperatures between 350 °C and 490 °C. At 350 °C, 450 °C, and 490 °C, they obtained phases of the types BiOI, Bi4O5I2, and Bi5O7I, respectively.
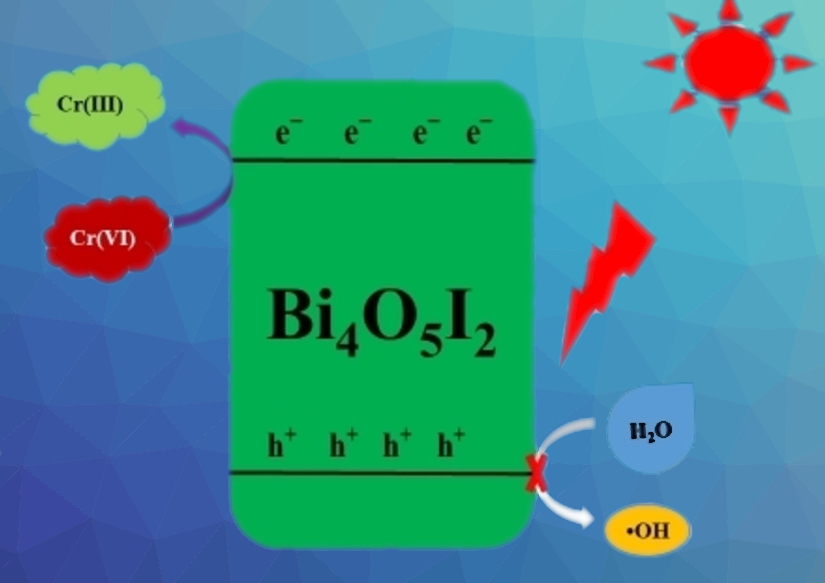
The highest photocatalytic efficiency for the reduction of hexavalent chromium under visible light irradiation was observed for BiOI calcined at 450 °C, i.e., for the phase Bi4O5I2. The team attributes this to the improved formation and transfer of charge carriers. When the visible light hits the photocatalyst, electrons in Bi4O5I2 are photo-excited to the conduction band, where they are responsible for the reduction of Cr(VI) to Cr(III) (pictured). The catalyst can be recovered and reused with only very small decreases in its photocatalytic efficiency.
- Photocatalytic Reduction of Hexavalent Chromium (Cr6+) Over BiOI Calcined at Different Temperature Under Visible Light Irradiation,
Adinas Oswald Kivyiro, Williams Kweku Darkwah, Robert Bofah‐Buoh, Desmond Ato Koomson, Masso Kody Christelle Sandrine, Joshua Buer Puplampu,
ChemistrySelect 2021.
https://doi.org/10.1002/slct.202101285