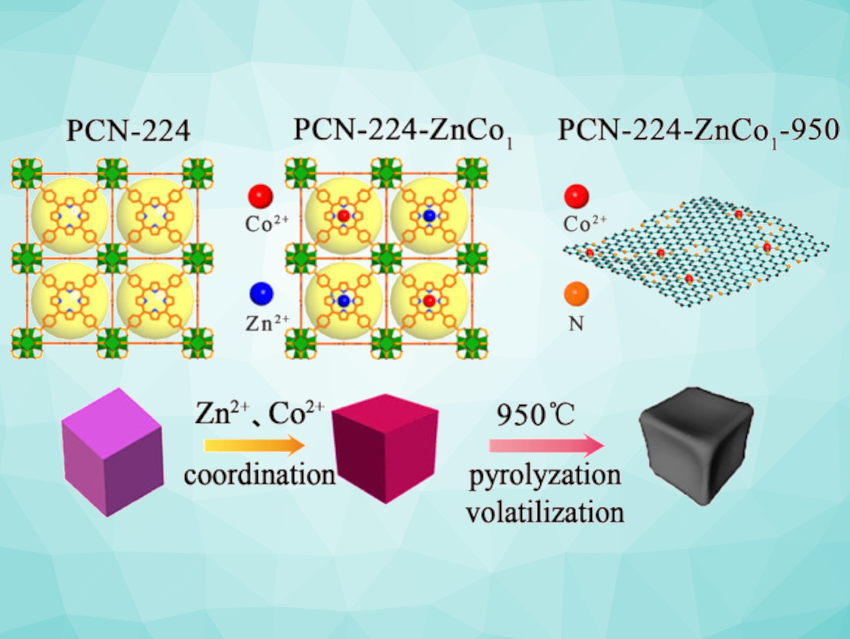
Cycloadditions of epoxides and CO2 provide an atom-economical path to cyclic carbonates. Metal-organic frameworks (MOFs) can serve as catalysts or catalyst precursors for conversions of CO2. PCN-224, for example, is a porphyrin-based MOF that can coordinate different metals in the porphyrin units. Coordinated cobalt can serve as a Lewis-acidic site that can promote the ring-opening of the epoxy reactants. However, it occupies the basic nitrogen site in the porphyrin, which weakens the absorption of CO2. Thus, balancing the acidic/basic site concentrations in the framework is important for developing effective catalysts.
Weijun Yang, Hunan University, Changsha, China, and colleagues have developed a catalyst for cycloadditions of epoxides and CO2 based on PCN-224 with tunable acidic/basic site concentrations. The team first synthesized PCN-224 without metals in the center of the porphyrin rings and then modified the MOF using ZnCl2 and CoCl2⋅6 H2O to obtain bimetal-functionalized PCN-224−ZnCo. Then, the bimetallic MOF was pyrolyzed at 950 °C to volatilize Zn (pictured), which partly frees up the basic nitrogen sites, produces activity-promoting defects, and increases the porosity.
The team found that the catalyst after pyrolysis contains well-dispersed Co2O3 nanoclusters in an N-doped porous carbon matrix. The acidic/basic site ratio of the catalyst can be tuned by varying the Zn/Co ratio in the precursor. The team found that a catalyst based on a PCN-224−ZnCo precursor with a Zn2+/Co2+ ratio of 1 :1 has suitable properties for carbon dioxide absorption and the efficient promotion of cycloadditions of CO2 with epoxides.
- Modulating the Acidic and Basic Site Concentration of Metal‐Organic Framework Derivatives to Promote the Carbon Dioxide Epoxidation Reaction,
Jinhua Ji, Hao Liu, Zewei Chen, Yajun Fu, Weijun Yang, Shuang‐Feng Yin,
Chem. Eur. J. 2021.
https://doi.org/10.1002/chem.202100430