Electrochemical water splitting is a promising process for converting the electricity generated from renewable sources into chemically stored energy. Much research effort has been dedicated to the development of cost-effective electrocatalysts for the two half-reactions of water splitting, i.e., the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER).
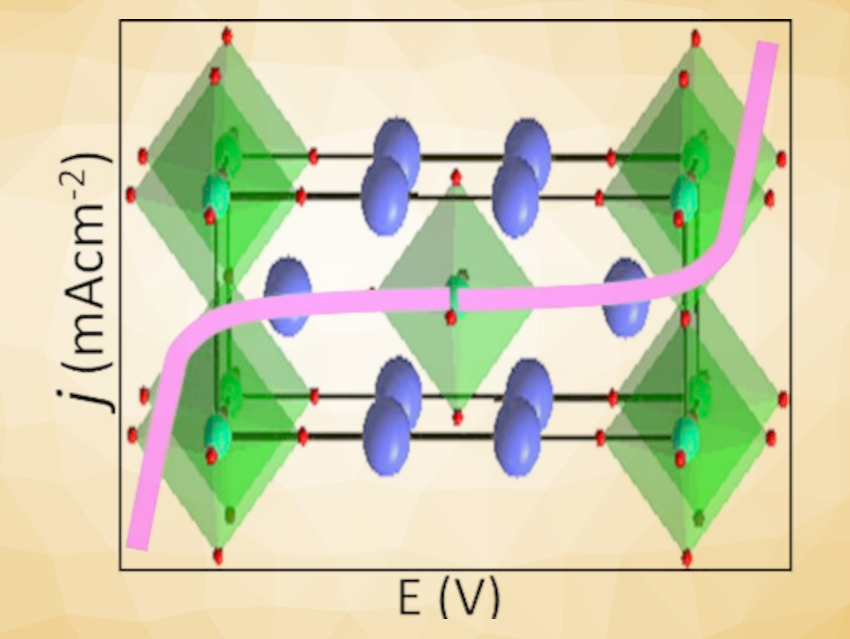
Md. Sofiul Alom and Farshid Ramezanipour, University of Louisville, KY, USA, have observed bifunctional electrocatalytic properties for water splitting in layered oxides of the type SrLaFe1-xCoxO4-δ (x = 0, 0.5, 1), which feature two-dimensional layers of octahedrally coordinated transition metals (pictured). These materials can catalyze both the OER and the HER. The three compounds, SrLaFeO4, SrLaCo0.5Fe0.5O4, and SrLaCoO4-δ, were synthesized via a solid-state method. Stoichiometric proportions of SrCO3, La2O3, Fe2O3, and Co3O4 were mixed, pressed into pellets, and then calcined at 1,300 °C in air for 72 h.
The third member of the series, SrLaCoO4-δ, shows the highest electrical charge transport and best electrocatalytic activity. It is also the only material in the series that has an oxygen deficiency, which could have a positive impact on the OER. An interesting feature of this work is the systematic enhancement of electrocatalytic properties as a function of the cobalt content, which can be attributed to its favorable electronic configuration.
- Layered Oxides SrLaFe1‐xCoxO4‐δ (x = 0–1) as Bifunctional Electrocatalysts for Water‐Splitting,
Md. Sofiul Alom, Farshid Ramezanipour,
ChemCatChem 2021.
https://doi.org/10.1002/cctc.202100867