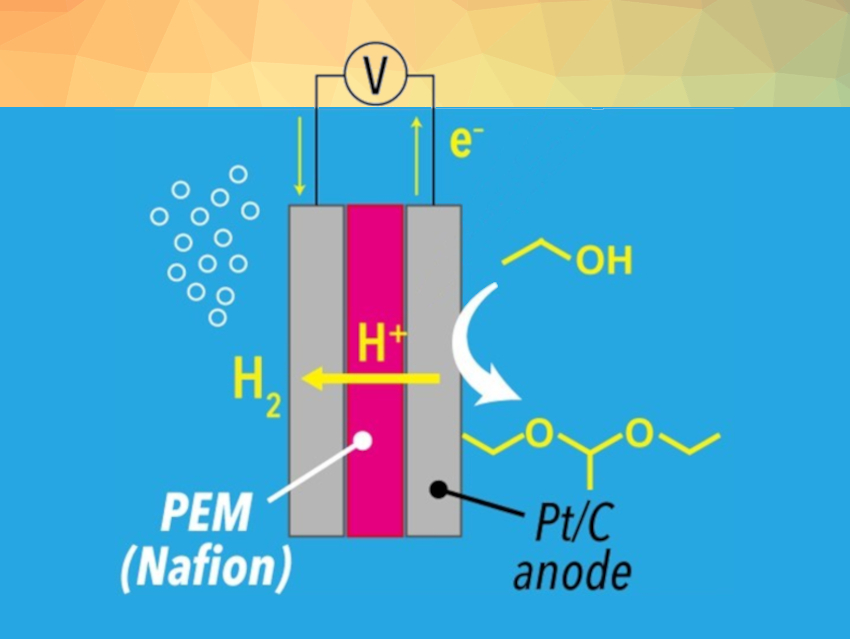
Electrochemistry can provide a sustainable approach for upgrading bioethanol to value-added chemicals. The proton exchange membrane (PEM) electrolysis of ethanol, for example, requires no chemical reagents and can be conducted using renewable electricity. These are desirable features from the viewpoint of green and sustainable chemistry.
1,1-Diethoxyethane (DEE), or acetaldehyde diethyl acetal, is a derivative of ethanol with applications in the chemical industry. For example, DEE can serve as an intermediate in the production of alkyl vinyl ethers, as a raw material in the fragrance industry, as an additive for diesel fuel, or as a biomass-derived solvent. DEE usually is produced by a two-step process from ethylene and ethanol.
Hitoshi Ogihara, Saitama University, Japan, and colleagues have developed a selective DEE synthesis from ethanol through PEM electrolysis (pictured above). DEE was produced through sequential electrochemical and non-electrochemical reactions: first, ethanol is electrochemically oxidized to acetaldehyde, and the Nafion membrane used as the PEM then serves as a solid acid catalyst for the acetalization of ethanol and acetaldehyde (pictured below).
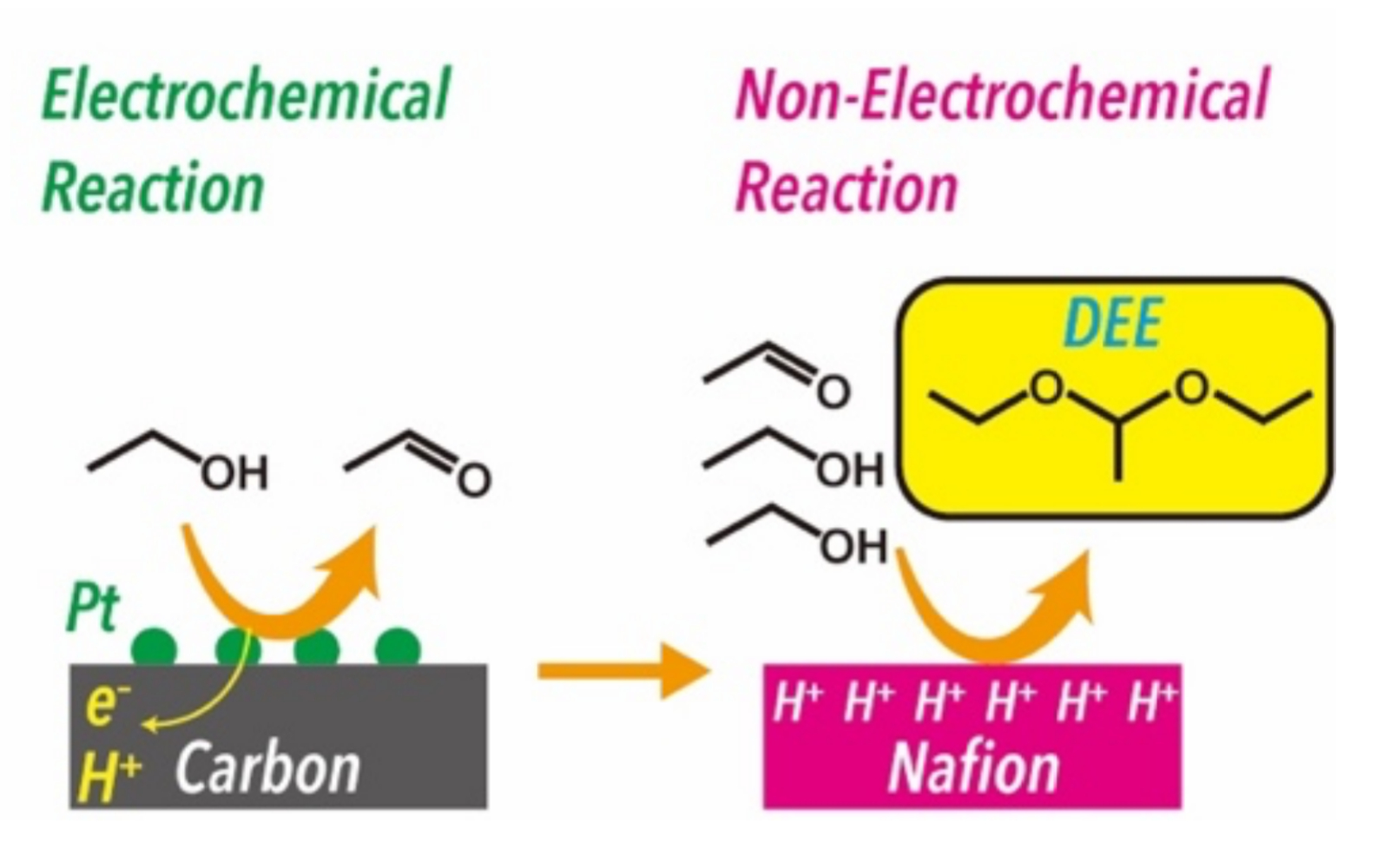
Electrolysis of pure ethanol yielded DEE with high faradaic efficiency (78 %). At the cathode, protons extracted from ethanol were reduced to hydrogen, indicating that the PEM electrolysis provides value-added products on both the anode (DEE) and the cathode (H2).
- Upgrading of Ethanol to 1,1-Diethoxyethane by Proton Exchange Membrane Electrolysis,
Daisuke Kawaguchi, Hitoshi Ogihara, Hideki Kurokawa,
ChemSusChem 2021.
https://doi.org/10.1002/cssc.202101188