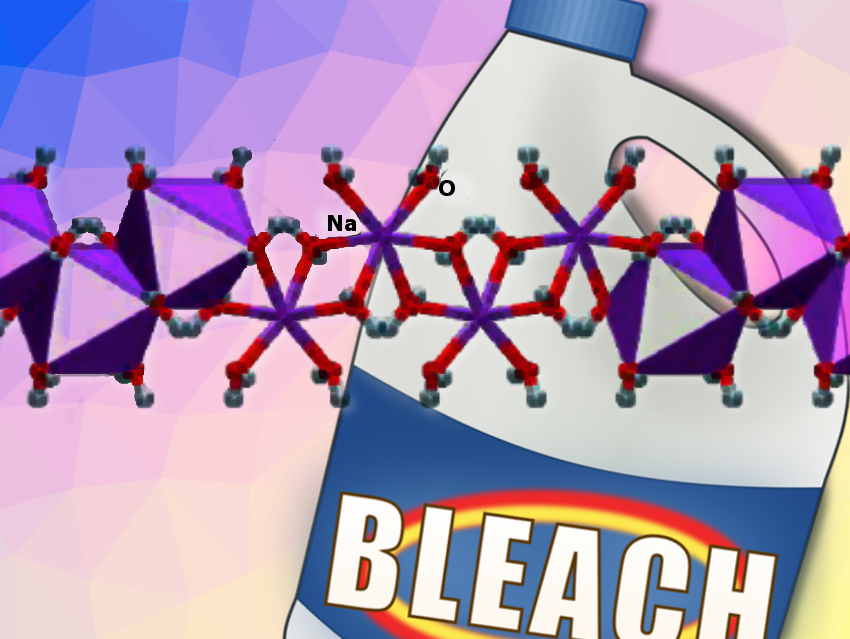
Despite the popularity of bleach as a household cleaner, and its importance as an industrial chemical that has been in manufacture for over 200 years, the solid-state structure of its active component has remained unknown. Known to chemists as sodium hypochlorite (NaOCl), bleach is often used dissolved in water (Clorox, Eau de Javel), but can also form colorless crystals.
Tomislav Friščić and co-workers, McGill University, Montreal, Canada, have determined the crystal structure of sodium hypochlorite bleach, as well as its bromine congener, sodium hypobromite, by X-ray diffraction. Both salts are representatives of hypohalites, a class of unstable compounds that are found in any inorganic chemistry textbook but, surprisingly, their structures have not been reported.
The result is the first direct structural observation of the hypochlorite and hypobromite ions. It is also the first observation of their supramolecular chemistry, particularly the ways in which they can interact with water. Given the historical significance and fundamental importance of hypohalites, this work solves a long-standing challenge and fills a surprising gap in basic chemistry knowledge.
Orthorhombic and Monoclinic Structures
A single crystal of NaOCl·5H2O was obtained from a commercial sample. X-ray diffraction revealed an orthorhombic unit cell.
The structure consists of alternating tapes of hydrated Na+ ions and of ClO− ions. The Na+-containing tapes have the composition [Na(H2O)4+]n. The cations are surrounded by six water molecules in the form of a distorted octahedron. Each cation is connected to two neighbouring ones by two pairs of bridging water molecules. The ClO−-containing tapes consist of a central chain of H2O molecules connected by O−H···O hydrogen bonds and lined by two sets of hydrogen-bonded ClO− ions. The ClO− and H2O molecules in each tape are associated with the surrounding Na+-containing tapes, acting as acceptors of long O−H···O hydrogen bonds. The ClO− ions in each tape are aligned in parallel and do not interact significantly.
Hypobromites are generally less stable than hypochlorites and are not commercially available. Samples of NaOBr·5H2O were prepared by adding Br2 to aqueous NaOH at −5 °C and storing at −20 °C.
Structure analysis revealed a monoclinic unit cell, space group P21/n. The structure of NaOBr·5H2O strongly resembles that of NaOCl·5H2O. The most significant difference is a shift in stacking of alternating cation- and anion-containing tapes, reflecting the change from orthorhombic to monoclinic symmetry.
The water-rich environment in solid NaOCl·5H2O and NaOBr·5H2O provided an opportunity or the team to investigate hydrogen-bonding behaviour of the hypohalites. The team found that constituent Cl and Br atoms do not participate in strong hydrogen bonding, resembling halogens in organic molecules.
- After 200 years: the structure of bleach and characterization of hypohalite ions by single‐crystal X‐ray diffraction,
Filip Topić, Joseph M. Marrett, Tristan H. Borchers, Hatem M. Titi, Christopher J. Barrett, Tomislav Friscic,
Angewandte Chemie International Edition 2021.
https://doi.org/10.1002/anie.202108843