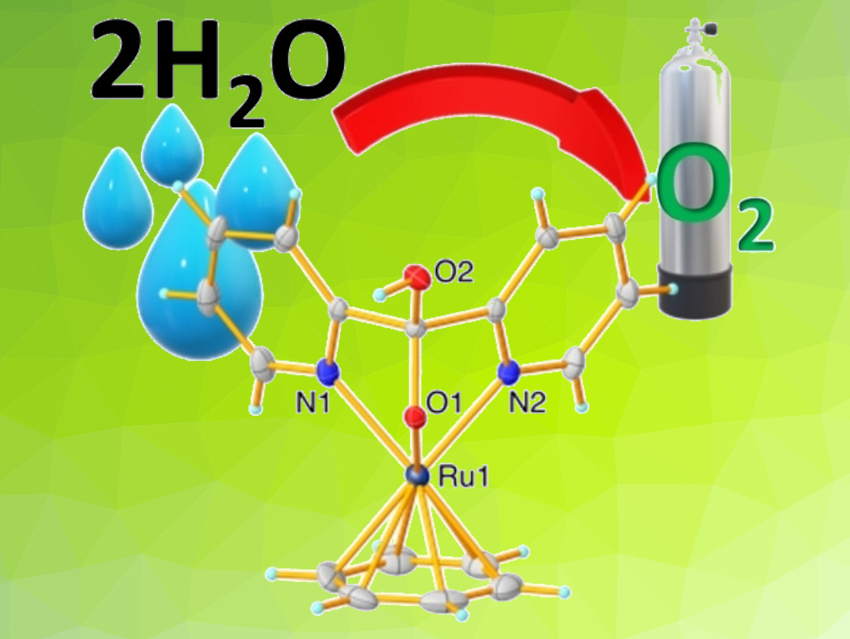
Designing molecular catalysts for water oxidation is an important part of the development of artificial photosynthesis as a sustainable path for splitting water into O2 and H2. Ruthenium-based catalysts are very efficient for water oxidation because of the stability of higher oxidation states of Ru and multi-electron transfer properties. A nucleophilic attack of water at a stable ruthenium oxo species is a promising route for the formation of the O–O bond required for O2 evolution.
Aditi Vatsa and Sumanta Kumar Padhi, Indian Institute of Technology (ISM) Dhanbad, Jharkhand, have developed a mono-cationic Ru half-sandwich complex, [Ru(η6-benzene)(hdm)]BF4 (Ru-hdm, pictured; hdm = hydroxydi(pyridine-2-yl)methanolate), as a catalyst for water oxidation. Using this complex, chemical water oxidation was performed in the presence of ceric ammonium nitrate (CAN) as an oxidant and electrochemical water oxidation was performed with 98 % Faradaic efficiency.
Ru-hdm was synthesized by reacting [Ru(η6-benzene)Cl2]2 with di-2-pyridylketone in the presence of NaOH and NaBF4 in water. In this approach, a nucleophilic attack of an hydroxide ion at the carbonyl group of di-2-pyridyl ketone generates the hdm ligand.
X-ray photoelectron spectroscopy (XPS) showed that the Ru-hdm complex acts as a pre-catalyst for the oxygen evolution reaction (OER) in the presence of CAN. The complex shows degradation in the reaction since the pH is lowered significantly after the addition of CAN. The team found that nanoparticles with [Ru(IV)=O] and [Ru(VI)=O] sites are formed in situ and could act as the active species for water oxidation under these conditions.
- Catalytic Water Oxidation by a RuII Half Sandwich Complex,
Aditi Vatsa, Sumanta Kumar Padhi,
Eur. J. Inorg. Chem. 2021.
https://doi.org/10.1002/ejic.202100481