The vicinal dihalogenation of alkenes is widely used in organic synthesis. To achieve this type of transformation, the alkene is often reacted with homonuclear (X2) or heteronuclear halogens (XY) in halogenated solvents. Most of these reagents are highly corrosive, toxic, and/or very reactive.
Pablo Barrio, Félix Rodríguez, Universidad de Oviedo, Spain, and colleagues have developed a sustainable alternative method that involves the treatment of the alkene with two easy-to-handle reagents—an N-halosuccinimide and a lithium halide—in the presence of acetic acid, using ethyl acetate as an environmentally benign solvent. The researchers propose that a strong interaction of the N-halosuccinimide (NXS) with linear H-bond aggregates formed from acetic acid and the presence of lithium halide (LiY) favors the formation of dihalogen species (pictured below), which can then react with the alkene.
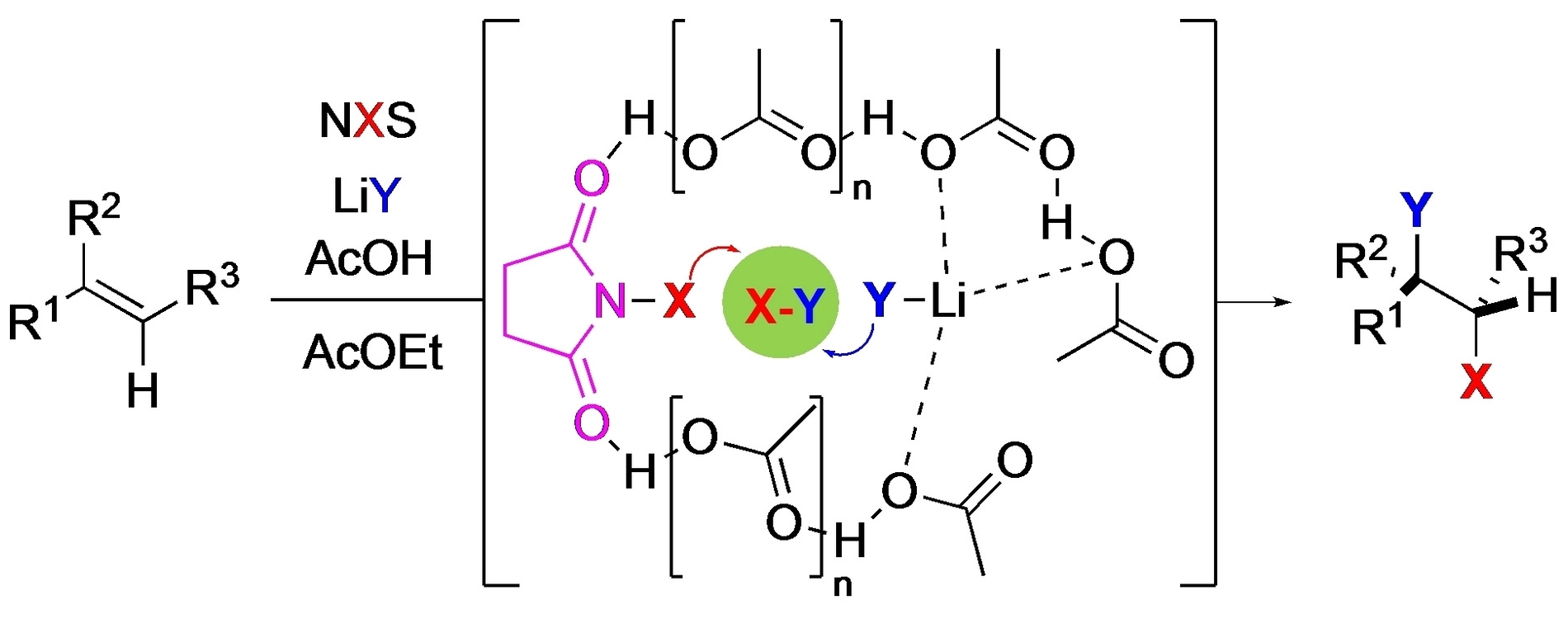
Pure products are usually obtained after a simple aqueous workup without a need for chromatography. The dihalogenation reaction is an alternative to the use of reagents such as ICl, IBr, BrCl, or Br2 and can also avoid the need for oxidants typically used for the in-situ generation of homonuclear diatomic halogens. The process allows both homonuclear and heteronuclear dihalogenations of a large range of alkenes.
- Dihalogenation of Alkenes Using Combinations of N‐Halosuccinimides and Alkali Metal Halides,
Rubén Rubio‐Presa, Olaya García‐Pedrero, Pablo López‐Matanza, Pablo Barrio, Félix Rodríguez,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100811



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)