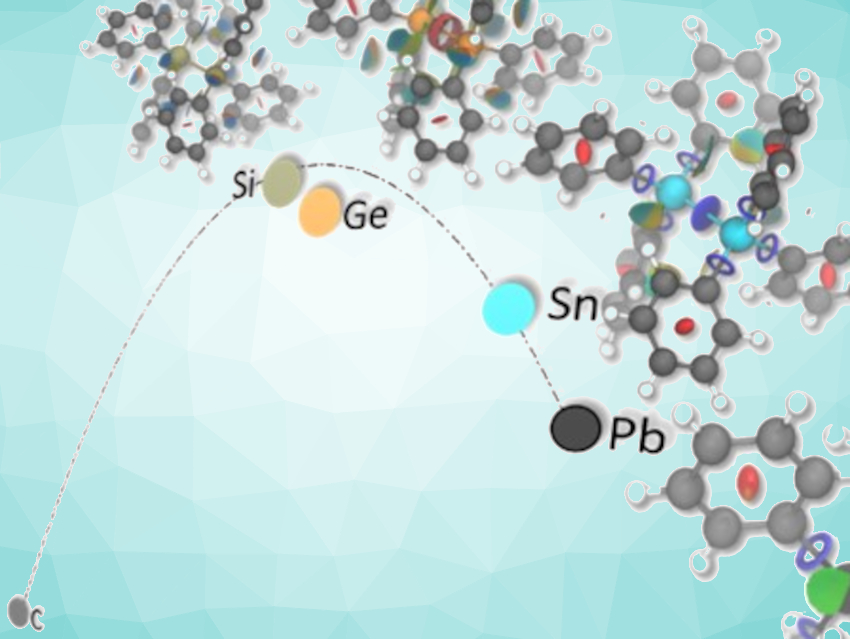
The concept that longer bonds are weaker and dissociate more easily is well-accepted in organic chemistry and sometimes used as the basis for estimating bond-dissociation energies (BDEs). This concept is based on early work on diatomic molecules. For larger structures, the situation is less clear, even though it is often assumed that there is a solid bond length–bond strength relationship.
Peter R. Schreiner, University of Gießen, Germany, and colleagues have challenged this view with quantum-chemical calculations that were used to study the BDEs of hexaphenylethane (Ph3C–CPh3) and its heavier group-14 (or tetrel) analogues. The higher tetrel derivatives are known and stable under ambient conditions—despite long central tetrel–tetrel bonds—but hexaphenylethane remains elusive. Energy decomposition analyses were used to rationalize the counterintuitive stabilities of the hexaphenylditetrels.
The team found that London dispersion interactions (a type of van der Waals force) are the decisive factor for the stability of heavier tetrel derivatives. The longer central bond length reduces phenyl/phenyl repulsion, while still allowing for substantial London dispersion stabilization. The work highlights the importance of noncovalent interactions for molecular aggregation and thermodynamic stability: Longer bonds do not necessarily have to be weaker in the presence of additional interactions around the bond in question.
- Hexaphenylditetrels – When Longer Bonds Provide Higher Stability,
Lars Rummel, Jan M. Schümann, Peter R. Schreiner,
Chem. Eur. J. 2021.
https://doi.org/10.1002/chem.202102271