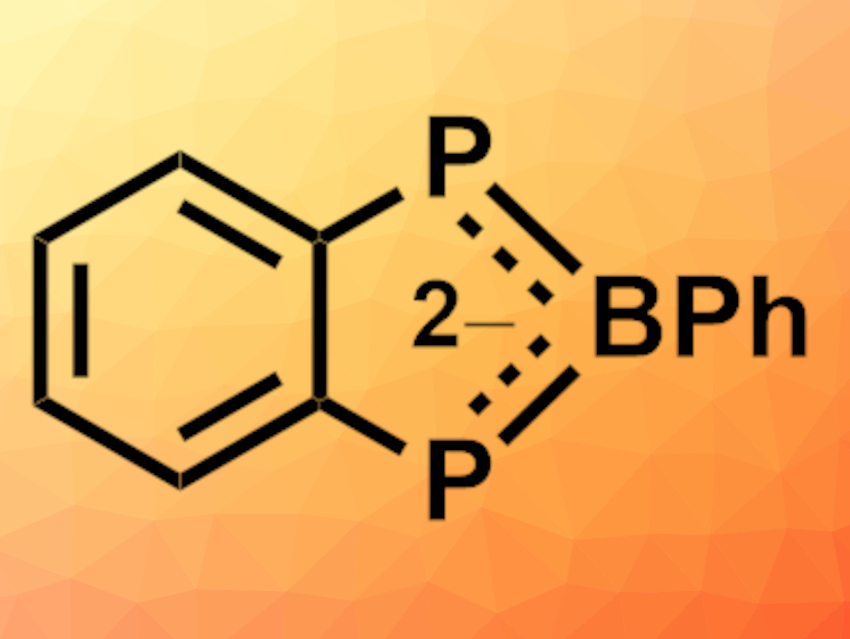
Aromatic, carbon-rich anions such as cyclopentadienyl can stabilize metal ions and allow the control of their electronic, magnetic, and chemical behavior. Analogues that contain phosphorus, nitrogen, or boron have similar uses, particularly for metals in lower oxidation states. Combining these elements in the same heterocycle is, thus, desirable. Boron/nitrogen analogues (azaborolides) have been studied, but boron/phosphorus analogues (phosphaborolides) had remained hypothetical so far.
Ian R. Crossley, University of Sussex, Brighton, UK, and colleagues have synthesized the first example of a diphosphaborolediide ion (pictured), which was isolated as its lithium salt. A one-pot sequential lithiation of bis(phosphino)benzene, quenched with PhBCl2 (pictured below), gave the benzodiphosphaborolediide salt in 50 % isolated yield. The dilithio salt could be obtained as either an oligomeric tetrahydrofuran (THF) solvate or a discrete tetramethylethylenediamine (TMEDA) adduct, both of which were characterized using X-ray diffraction.
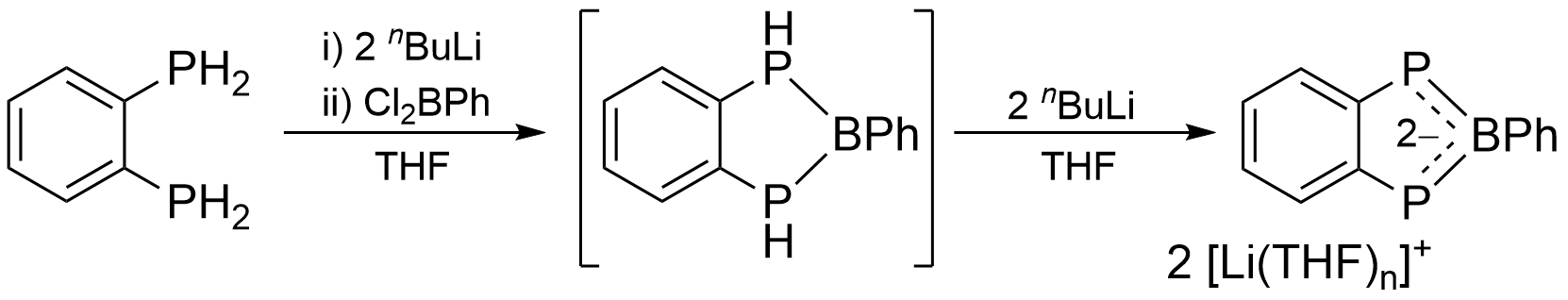
Density functional theory (DFT) calculations confirmed the aromaticity of the diphosphaborolediide. It can coordinate as a π-ligand to zero-valent molybdenum, while also serving as a bridging σ-ligand through its phosphorus lone pairs. Benzodiphosphaborolediides could, thus, be versatile additions to the “hetero-cyclopentadienyl” ligand family.
- A Benzodiphosphaborolediide,
Kyle G. Pearce, Elinor P. F. Canham, John F. Nixon, Ian R. Crossley,
Chem. Eur. J. 2021.
https://doi.org/10.1002/chem.202103427