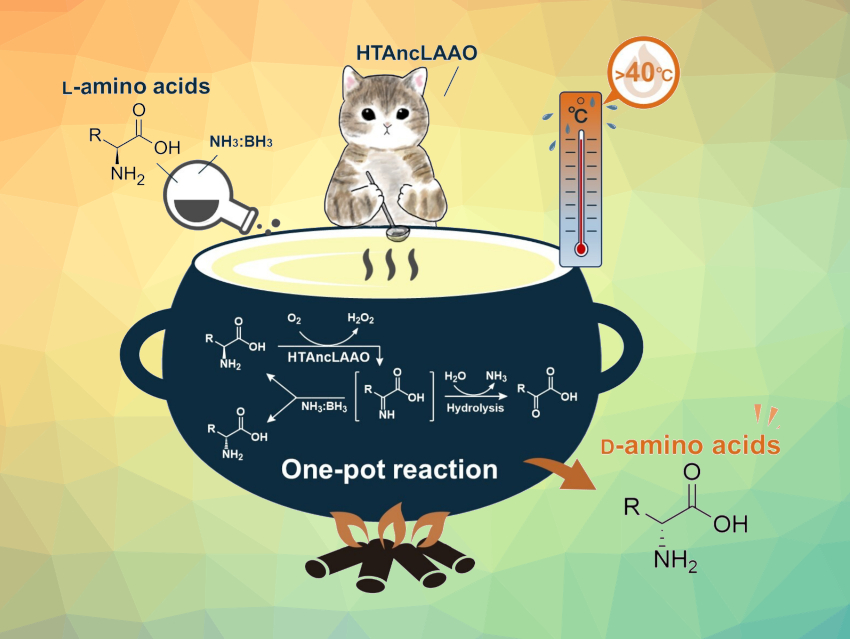
Enantiomerically pure amino acids can be useful precursors for fine chemicals, such as pharmaceutically active compounds and pesticides. L-amino acid oxidases (LAAOs) are enzymes that can be used in the deracemization of D,L-amino acids to obtain D-enantiomers in their pure form.
Sohei Ito, University of Shizuoka, Japan, Shogo Nakano, University of Shizuoka and Japan Science and Technology Agency, Saitama, and colleagues have designed a hyper-thermostable ancestral LAAO (HTAncLAAO) using a combination of sequence data mining and ancestral sequence reconstruction. The enzyme was then produced using E. coli bacteria. HTAncLAAO exhibits extremely high thermal stability and better long-term stability compared with conventional LAAOs.
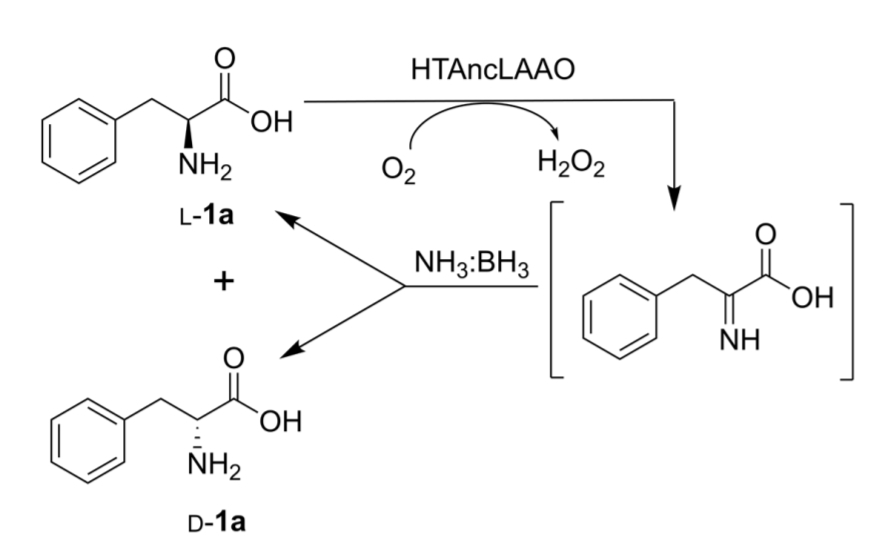
Optically pure D-amino acids (>99 % ee), such as 3-fluoro D-phenylalanine, 4-nitro-D-phenylalanine, and D-isoleucine were synthesized from their racemates at preparative scale. HTAncLAAO and ammonia borane were utilized as biocatalyst and reductant, respectively. HTAncLAAO was used to oxidize only the L-amino acid to the corresponding imino acid (pictured), and ammonia borane to reduce the imino acid. This leads to deracemization. The reaction proceeds at 40 °C. The results indicate that HTAncLAAO is a suitable biocatalyst to perform the deracemization of racemic amino acids to the D-enantiomer.
- Reconstruction of hyper‐thermostable ancestral L‐amino acid oxidase to perform deracemization to D‐amino acids,
Chiharu Ishida, Ryo Miyata, Fumihito Hasebe, Azusa Miyata, Shigenori Kumazawa, Sohei Ito, Shogo Nakano,
ChemCatChem 2021.
https://doi.org/10.1002/cctc.202101296