Conductive metal–organic framework (MOF) materials are regarded as promising oxygen electrocatalysts. However, they have poor catalytic activity because they have a limited number of exposed active sites. Fabricating conductive MOFs with a hollow structure and abundant active sites remains challenging.
Xiong Wen (David) Lou, Nanyang Technological University, Singapore, and colleagues have developed a successive cation and ligand exchange method to synthesize cobalt–copper–based bimetal–organic framework nanoboxes (CoCu-MOF NBs). The team used tannic acid (TA) to etch the MOF and promote a cation exchange process in a Cu2+ solution.
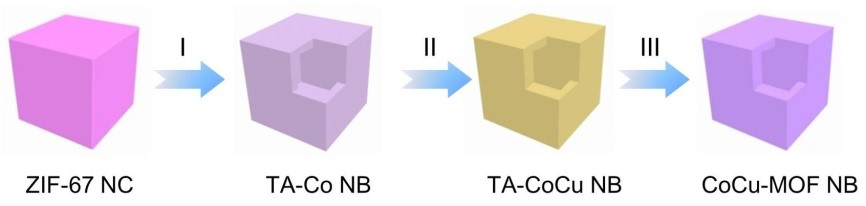
Co-based zeolitic imidazolate framework-67 nanocubes (designated as ZIF-67 NCs; pictured above) with a size of about 1.5 μm are used as the starting material. The initial ZIF-67 NCs were converted into tannic-acid-chelated Co/Cu complex nanoboxes (TA-CoCu NBs) through a chemical etching process. TA-Co NBs are transformed into Cu-modified TA-Co NBs (TA-CoCu NBs) via a cation exchange process in a Cu2+ ion solution. Here partial Co sites of TA-Co NBs are replaced by Cu atoms. Subsequently, the TA-CoCu NBs are transformed into the final CoCu-MOF NBs via a ligand exchange process. During this process, the TA linkers of TA-CoCu NBs are gradually replaced with 2,3,6,7,10,11-hexahydroxytriphenylene (HHTP) ligands, likely due to the stronger chelating ability of HHTP ligands relative to the TA molecules.
The CoCu-MOF NBs, which bear active cobalt–copper oxyhydroxide groups on their surface, show good performance in the electrocatalytic oxygen evolution reaction (OER) with a small overpotential of 271 mV at 10 mA cm–2 and a high turnover frequency of 0.326 s–1 at an overpotential of 300 mV.
Synchrotron radiation studies revealed that the post-formed CoCu-based oxyhydroxide analog is the actual active structure for CoCu-MOF NBs toward efficient OER. Theoretical calculations also show that the synergetic electronic interactions of Co and neighboring Cu sites in CoCu-MOF NBs are favorable for generating key oxo-intermediates toward fast OER kinetics. According to the researchers, these findings could provide novel insights for the rational design and synthesis of efficient oxygen catalysts for energy-related applications.
- Synergetic Cobalt‐Copper‐Based Bimetal‐Organic Framework Nanoboxes toward Efficient Electrochemical Oxygen Evolution,
Weiren Cheng, Zhi-Peng Wu, Deyan Luan, Shuang-Quan Zang, Xiong Wen (David) Lou,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202112775