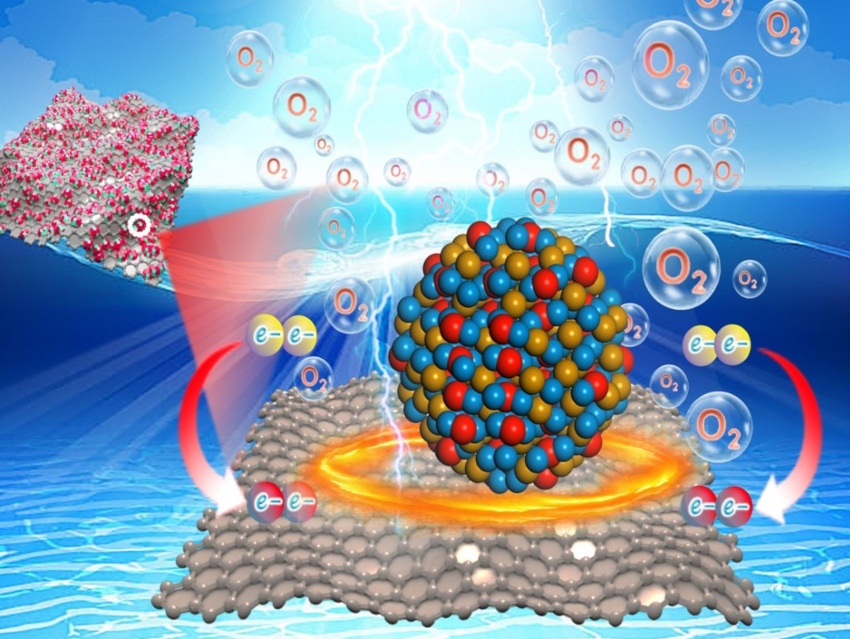
Achieving a highly efficient electrochemical oxygen evolution reaction (OER) is important for the use of electrolytic water splitting as a part of sustainable energy technologies. Great efforts have been made to advance the OER activity of catalysts, but the effects of carbon supports on the electrocatalytic activity still warrant further investigation.
Zhimin Chen, Zhiyu Ren, Heilongjiang University, Harbin, China, and colleagues have synthesized nickel-iron (hydr)oxide nanoparticles (NiFeO NPs) on carbon supports. The preparation began with the electrostatic assembly of metal ions (Ni2+ and Fe2+) and abundant oxygen-containing functional groups (e.g., hydroxy-, epoxide-, carboxy groups) on the surface of graphene oxide (GO). A subsequent reduction, accompanied by the conversion from GO into rGO, converted the Ni and Fe species on the surface to NiFeO NPs.
The team found that, due to strong electrostatic coupling, ultrasmall NiFeO NPs (≈ 2 nm) produced during the in-situ reduction process were firmly anchored on the surface of reduced graphene oxide (rGO). This strong interaction causes an electronic modulation of the active metal centers and accelerates the charge transfer. The OER activity of NiFeO NPs anchored on rGO was significantly improved, with an overpotential as small as 201 mV at 10 mA cm–2 and remarkable stability.
An electrolyzer using NiFeO NPs/rGO as the anode and commercial Pt/C as the cathode could be powered by a solar cell for efficient alkaline seawater splitting. This work could provide a path to further improvements of the intrinsic activity of energy-related electrocatalytic materials.
- Promoting Electrocatalytic Oxygen Evolution of Ultrasmall NiFe (Hydr)oxide Nanoparticles by Graphene‐Support Effects,
Shuang Liu, Erping Cao, Zhimin Chen, Hao Wu, Bowen Liu, Jing Yang, Shichao Du, Zhiyu Ren,
ChemSusChem 2021.
https://doi.org/10.1002/cssc.202101913