CO2 capture and utilization (CCU) could help to mitigate greenhouse gas emissions. The electrochemical reduction of CO2 using renewable electricity is an attractive CCU approach for the production of value-added chemical feedstocks and fuels, e.g., methane.
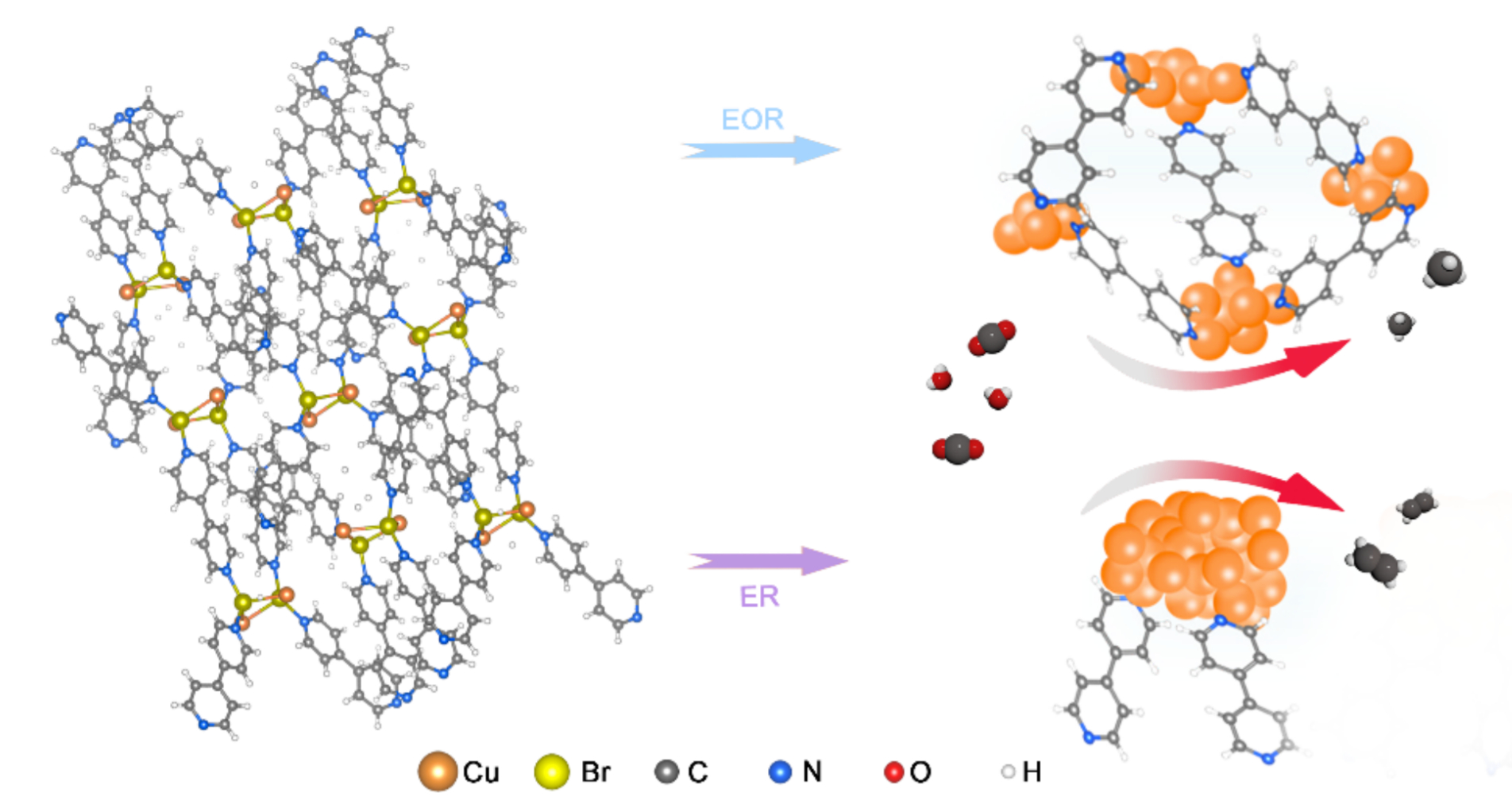
During electrochemical CO2 reduction on copper, a decrease of the coordination number of copper or a smaller size of copper nanoparticles is beneficial for the catalytic activity. However, it is challenging to stabilize such highly active Cu sites because they are prone to aggregation. The direct electrochemical reduction (ER, pictured below, violet arrow) of Cu-based metal-organic frameworks (MOFs), for example, results in comparatively large Cu nanoparticles.
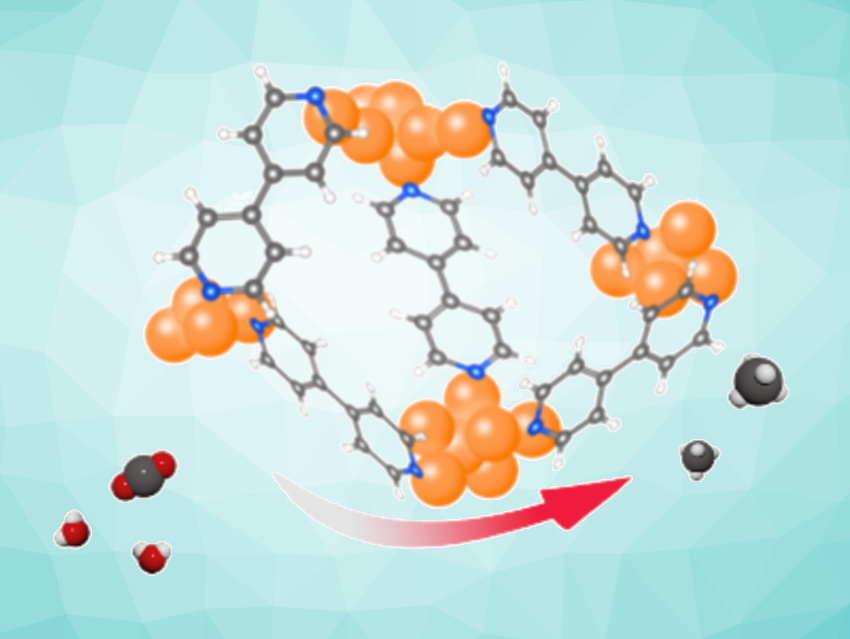
Jie Zeng, Zhigang Geng, University of Science and Technology of China, Hefei, Fengwang Li, University of Sydney, Australia, and colleagues have developed an electrochemical oxidation–reduction (EOR) pretreatment method to prepare sub-nanometer Cu clusters in situ from Cu-based metal–organic frameworks (MOFs). The team used cycling potentials to induce oxidation and reduction processes in a MOF of the type Cu(bipy)Br (bipy = 4,4′-bipyridine) to transform it into Cu nanoclusters with sizes below 1 nm (pictured below, blue arrow).
The resulting catalyst shows a faradaic efficiency (FE) of 51.2 % for CH4 with a partial current density of over 150 mA cm–2. The FE for CH4 remained above 45 % throughout a nine-hour electrolysis test at a constant current density of 300 mA cm–2. The team found that the highly active Cu nanoclusters are coordinated and stabilized by residual bipyridine ligands.
- Molecular stabilization of sub‐nanometer Cu clusters for selective CO2 electro‐methanation,
Jie Zeng, Han Zhang, Yu Yang, Yongxiang Liang, Jun Li, An Zhang, Han Zheng, Zhigang Geng, Fengwang Li,
ChemSusChem 2021.
https://doi.org/10.1002/cssc.202102010