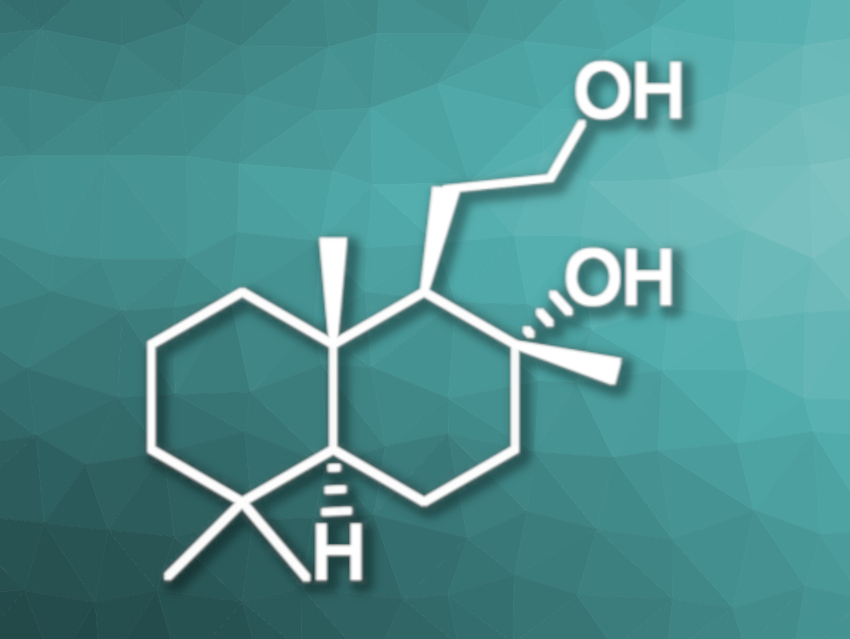
Ambergris, which is produced in the digestive tract of the sperm whale, has been used in perfumery for a long time. To avoid the hunting and exploitation of whales, synthetical alternatives have been developed. One of the components of ambergris is Ambrox® (pictured below).
One route for the synthesis of (−)-ambrox is a three-step synthesis from (–)-sclareol. An oxidative degradation of the side chain of (−)-sclareol leads to (+)-sclareolide (pictured below), then (+)-sclareolide is reduced to (–)-ambradiol, and (–)-ambradiol is converted into (–)-ambrox. Traditionally, the reduction of (+)-sclareolide is performed using superstoichiometric amounts of reducing agents such as KBH4 or LiAlH4, resulting in large amounts of waste during the work-up.
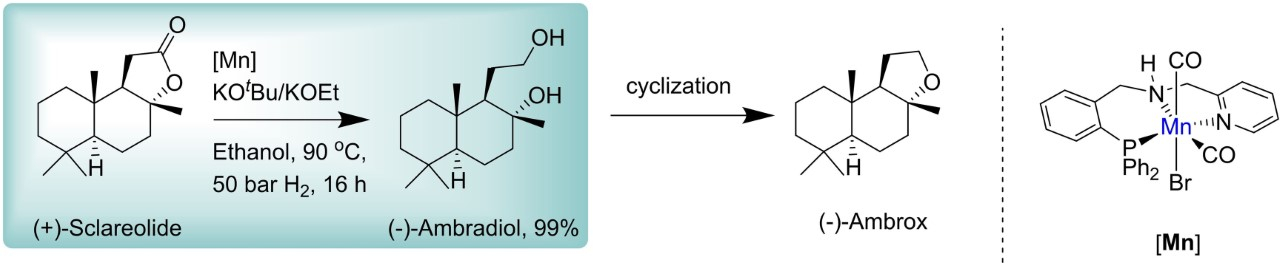
Thomas Schaub, Catalysis Research Laboratory (CaRLa), University of Heidelberg, Germany, and BASF SE, Ludwigshafen, Germany, and colleagues have developed a protocol for the hydrogenation of (+)-sclareolide to (–)-ambradiol catalyzed by a manganese pincer complex (pictured above). The team used KOtBu or KOEt as the base and ethanol as the solvent, and the reaction was performed under 50 bar H2 at 90 °C.
The reaction proceeds under relatively mild conditions at low manganese (0.1 mol%) and base loadings (1–2 mol%) using an air- and moisture-stable manganese catalyst bearing a relatively easy-to-synthesize pincer ligand. This approach offers a more economic and sustainable path to ambradiol without any stoichiometric waste or the use of precious-metal catalysts.
- Manganese‐Catalyzed Hydrogenation of Sclareolide to Ambradiol,
Viktoriia Zubar, Niels Lichtenberger, Mathias Schelwies, Thomas Oeser, A. Stephen K. Hashmi, Thomas Schaub,
ChemCatChem 2021.
https://doi.org/10.1002/cctc.202101443