Flow chemistry is a useful technology, both in academic settings and in applications such as the industrial-scale manufacturing of pharmaceuticals. Due to the increased reagent exposure to light when compared with batch chemistry, flow chemistry is particularly well-suited to photoredox catalysis and photochemical reactions in general.
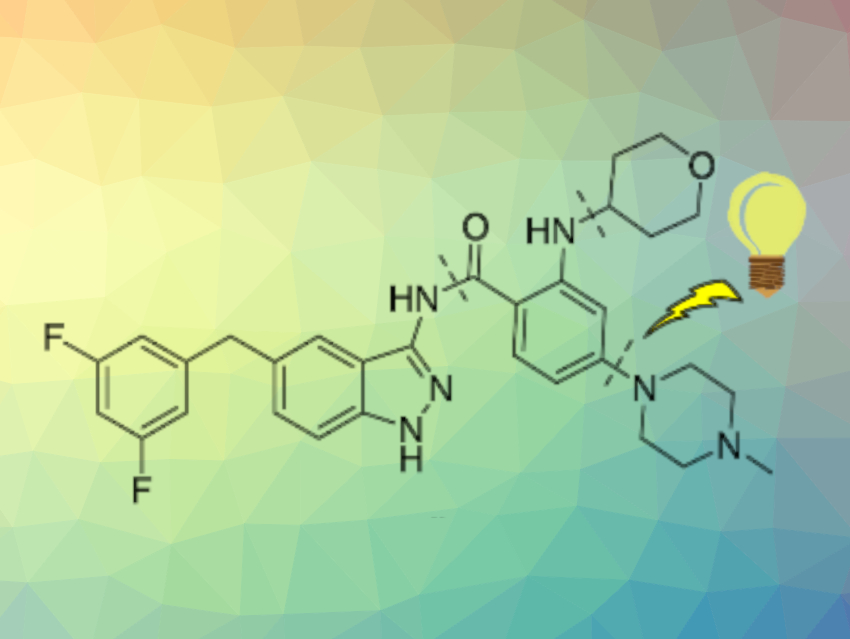
John G. Riley and colleagues, BioVectra, Charlottetown, Canada, have performed a total synthesis of entrectinib, a therapeutic for non-small cell lung cancers and solid tumors (marketed as Rozlytrek), using photoredox catalysis in flow. The total synthesis of this drug is based on a NiBr2-mediated C–N photoredox coupling as a key step. An evaluation of multiple intermediates for their use in this critical step in flow showed that electron-rich substrates lead to hydro-dehalogenation, while electron-deficient aryl halides achieve higher conversions to the desired C–N coupling product.
With the best substrate for the C–N coupling discovered, the remainder of the synthesis to entrectinib was performed via an hydrolysis step, a coupling with an aminoimidazole, and deprotection. The step count in the overall synthesis was reduced from nine in previous processes to six. According to the researchers, the process is scalable and the work provides useful insight into pharmaceutically relevant C–N photoredox coupling reactions in flow.
- Total synthesis of entrectinib with key photo‐redox mediated cross‐coupling in flow,
Morgan J. Cordell, Matt Rhodes Adams, Jean-Francois Vincent-Rocan, John G. Riley,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202101143