Benzene and cyclobutadiene are typical aromatic and antiaromatic systems, respectively. Polycyclic conjugated scaffolds combining both benzenoid and cyclobutadienoid rings have interesting electronic structures that are governed by both aromaticity and antiaromaticity.
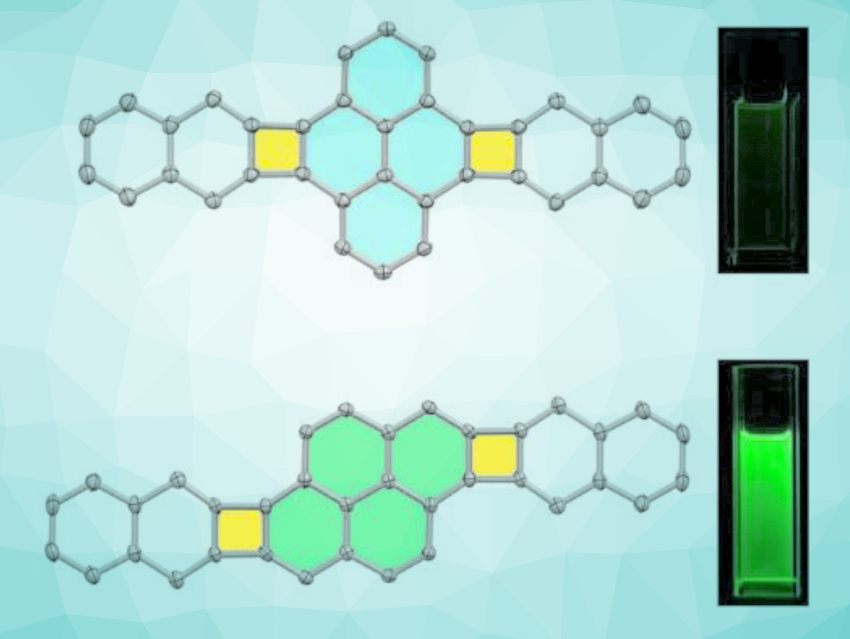
Qian Miao and colleagues, Chinese University of Hong Kong, Hong Kong SAR, China, have synthesized new polycyclic conjugated scaffolds (pictured) that have two cyclobutadienoids fused to a central pyrene subunit with two different fusion patterns. The compounds were prepared from dibromopyrene derivatives and different 1,4-dialkyl-benzooxanorbornadienes via a palladium-catalyzed annulation, followed by aromatization. The products were characterized using single-crystal X-ray crystallography.
The two regioisomeric polycyclic conjugated scaffolds show different local aromaticity and photophysical properties as a result of the different fusion patterns of the pyrene and cyclobutadienoid units. The team found that the π-stacking of the bisnaphtho[2’,3’:3,4]cyclobuta[1,2-e:1’,2’-l]pyrene-type scaffold can be tuned by changing the length of the attached alkyl substituents. The derivative with the largest π-overlap could be useful as a p-type organic semiconductor in solution-processed thin-film transistors.
- Synthesis, Structures and Properties of Bis(naphthocyclobuta)pyrenes,
Man Gao, Han Chen, Qian Miao,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202101315



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)