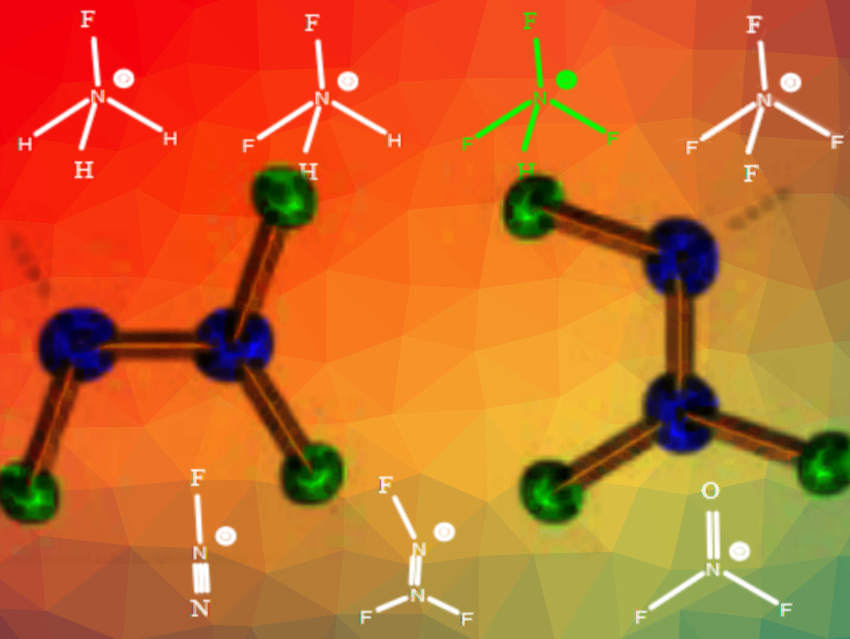
Fluoro-nitrogen cations are useful oxidizers, high energy density materials, ingredients in NF3/F2 gas generators for chemical lasers, rocket propellants, and electrophilic fluorinating agents. However, little is known about the exact structure and bonding of the simplest members of this class of compounds.
Karl O. Christe, University of Southern California, Los Angeles, USA, and colleagues have determined the crystal structures and bonding of NH3F+, NH2F2+, and N2F3+. NH3F+ was prepared by fluorination of carbamates and the difluoroammonium cation through protonation of NHF2 or FN3. N2F3+ antimony salts were obtained from N2F4 and SbF5 in anhydrous HF solution. The cations are challenging to prepare as some compounds are explosive, they are difficult to handle, and their crystal structures are prone to disorder.
The team found that in the series N2F+, N2F3+, NF2O+, NH3F+, NH2F2+, and NF4+, the hybridization of the central nitrogen atom and the number of fluorine substituents are the key factors influencing the N-F bond lengths. The N-F bond length decreases from 1.40 Å to 1.26 Å with increasing fluorine substitution and increasing s-characterof the nitrogen atom. High-level quantum chemical calculations were used to support these conclusions. The team claims that the structure–bonding relationships contrast with prior reports because the bonding in the cations is dominated by the s-character of the nitrogen atom and the number of fluorine substituents.
- Fluoro‐nitrogen Cations,
Karl O. Christe, Ashwani Vij, William W. Wilson, Ralf Haiges, Kyle C. Edwards, David A. Dixon,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202116565