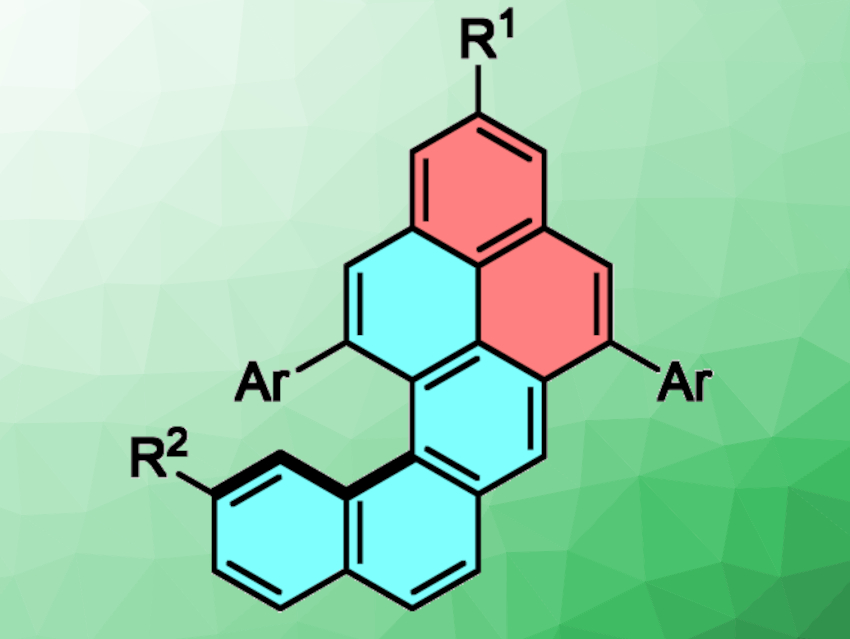
Nanographenes, i.e., substructures of graphene, are interesting research targets due to the synthetic challenges they pose and their possible applications in optical and electronic devices. Helicenes, for example, have ortho-fused benzene rings and constitute a class of nanographenes. The lateral expansion of helicene π-systems can lead to nanographenes with interesting chiroptical properties. Developing a simple method for the functionalization of such helicene-like compounds could be useful for the development of chiral nanographene materials, e.g., for applications in organic electronics.
Wesley A. Chalifoux, University of Nevada, Reno, USA, and colleagues have developed an efficient synthesis of functionalized naphtho[1,2-a]pyrenes (pictured above). The team used an indium-chloride- and silver-bistriflimide-catalyzed two-fold alkyne benzannulation of diyne intermediates (pictured below). A variety of naphtho[1,2-a]pyrene derivatives were synthesized in moderate to excellent yields.
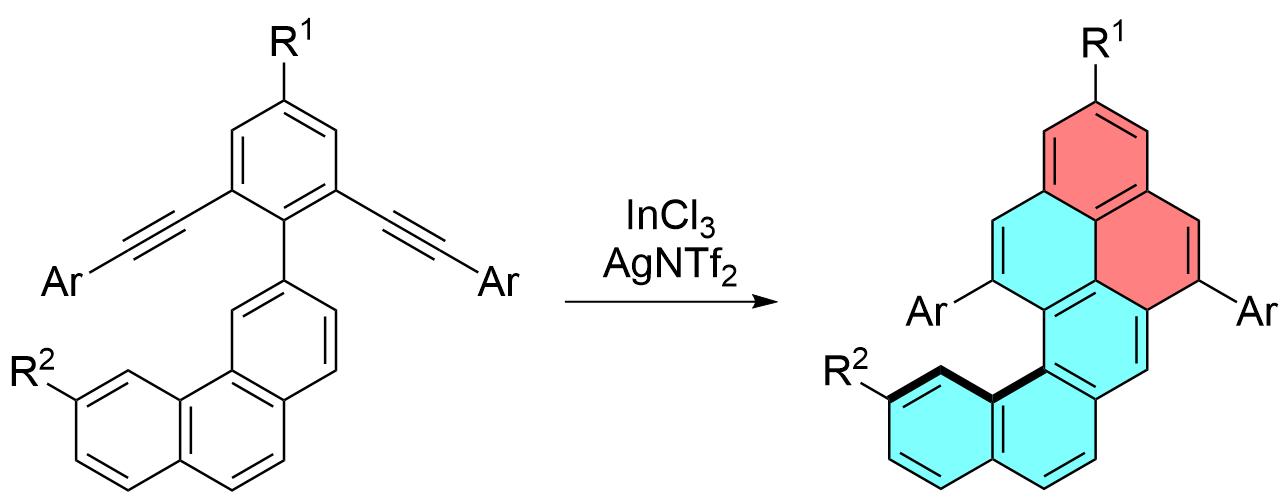
The compounds were analyzed using IR and Raman spectroscopy, and enantiomeric energy barriers were determined using variable-temperature dynamic high-performance liquid chromatography (DHPLC). The team found that functionalization in the “cove” region of the molecules increases the energy barrier for enantiomerization due to steric repulsion. This allowed them to separate enantiomers using HPLC and study them using circular dichroism (CD) spectroscopy. The researchers propose that the synthetic approach could be used to access larger π-expanded helicene-like nanographene compounds.
- π‐Extended Helical Nanographenes: Synthesis and Photophysical Properties of Naphtho[1,2‐a]pyrenes,
Wesley A. Chalifoux, Paban Sitaula, Ryan J. Malone, Giovanna Longhi, Sergio Abbate, Eva Gualtieri, Andrea Lucotti, Matteo Tommasini, Roberta Franzini, Claudio Villani, Vincent J. Catalano,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202101466