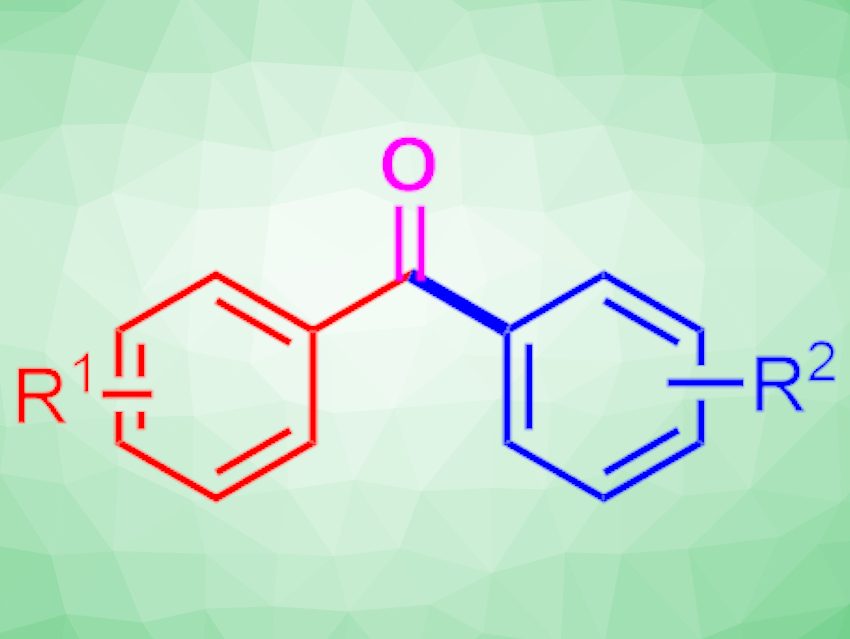
The Friedel–Crafts reaction is a useful method for constructing C–C bonds at aromatic positions. It allows the construction of valuable, complex organic compounds. However, the reaction typically requires pre-activated substrates, which must be synthesized in advance, and corrosive reaction conditions.
Frederic W. Patureau and colleagues, RWTH Aachen University, Germany, have used an electrochemical strategy to directly activate and oxidize simple, relatively inert methylarenes, which then undergo C–C bond formation with a broad range of arenes to produce the corresponding unsymmetrical phenones (reaction pictured below). The team used a graphite anode, an aluminium anode, hexafluoro-2-propanol (HFIP) as the solvent, and sodium dodecylbenzenesulfonate as the electrolyte. They also added a small amount of water.
.jpg)
The team found that the oxygen atom of the phenone products comes from water, which allows labeling with isotopes such as 17O. The method provides easy and direct access to valuable phenones, e.g., Ketoprofen-derived anti-inflammatory drug candidates. According to the researchers, this new synthetic method offers a more direct, atom- and step-efficient alternative to traditional Friedel–Crafts or Suzuki–Miyaura-derived acylation reactions.
- Direct Dehydrogenative Access to Unsymmetrical Phenones,
Congjun Yu, Raolin Huang, Frederic W. Patureau,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202201142