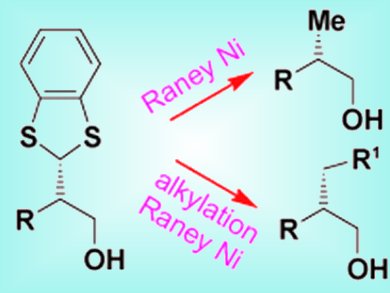
The α alkylation of aldehydes is considered the “Holy Grail” of organocatalysis. Pier Giorgio Cozzi and colleagues, University of Bologna, Italy, have now reported the first practical and highly stereoselective α addition of a formyl group to aldehydes. They used commercially available benzodithiolylium tetrafluoborate to introduce a 1,3-benzodithiol group which could be metalated with nBuLi or reduced with Raney Ni to give easy access to the methyl group.
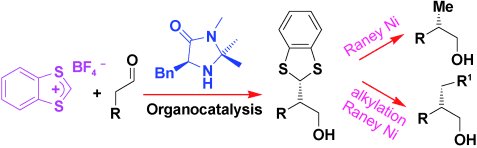
The team found that the nature of the base was crucial for the reaction; organic bases resulted in poor yields because of side reactions. Inorganic bases were more suitable, in particular NaH2PO4 was found to give the best yields. The reaction was tolerant of a large variety of functional groups such as chloro and cyano groups, and amides and acetals, giving yields up to 95 %. The enantiomeric excesses obtained were in the range 92–97 %.
- Highly Enantioselective α Alkylation of Aldehydes with 1,3-Benzodithiolylium Tetrafluoroborate: A Formal Organocatalytic α Alkylation of Aldehydes by the Carbenium Ion
A. Gualandi, E. Emer, M. G. Capdevila, P. G. Cozzi,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201102562