The functionalization of alkenes with selenium compounds offers attractive possibilities for synthetic organic chemistry. Selenium electrophiles are quite powerful and can easily react with double bonds to generate the corresponding addition products after reaction with a nucleophile.
Thomas Wirth, Cardiff University, UK, and colleagues describe new chiral diselenides prepared from readily available, cheap starting materials in a very short synthesis and the addition reactions of their corresponding selenium electrophiles to alkenes.
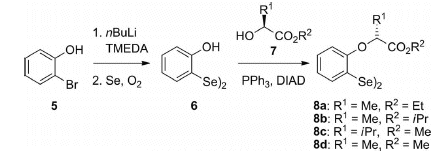
The starting material for the one-step synthesis is bis(2-hydroxyphenyl) diselenide (6), which is easily prepared from
2-bromophenol (5). A subsequent Mitsunobu reaction can be successfully used with different chiral alcohols 7 under
very mild conditions to generate the required diselenides 8 in high yields.
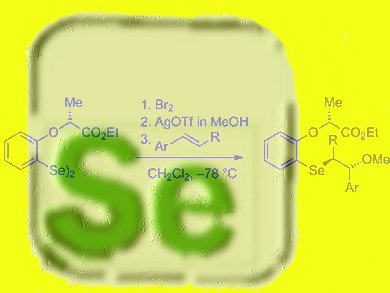
The selenium electrophiles have been successfully applied in methoxyselenenylation reactions with good yields and diastereomeric ratios.
Image: (c) Wiley-VCH
- Asymmetric Methoxyselenenylations with Chiral Selenium Electrophiles,
Liwei Zhao, Zhong Li, Thomas Wirth,
Europ. J. Org. Chem. 2011.
DOI: 10.1002/ejoc.201101373



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)