Porous Iron Oxide Material Effectively Removes Ozone From Air
When automobile traffic moves through the summer heat, ozone alerts lead to driving bans in some countries, such as Germany. Ozone is formed when nitrogen oxide from exhausts is irradiated with UV light and releases an oxygen atom, which reacts with an oxygen molecule to form an ozone molecule. A number of electrical devices, such as photocopiers and laser printers, also release this unhealthy gas. A team led by Thomas Mathew and Kenichirou Suzuki, Toyota Central R&D Labs Inc., Japan, report how they have now developed an iron oxide containing material that could lead to a new generation of ozone filters.
Ozone is a very dangerous air pollutant, irritating the airway and eyes as well as causing headaches. It also hinders plant growth and is believed to play a role in the die-off of forests. The typical “ozone smell” when using older photocopiers and laser printers is not the ozone itself: it is reaction products from the ozone. Most newer devices are equipped with filters that convert the ozone. Removal of O3 is essential for aircraft applications because O3 is an unavoidable pollutant at high altitude and can be introduced into the aircraft cabins during the flight.
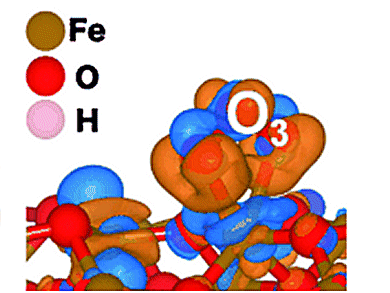
Previous materials for the elimination of ozone have a number of disadvantages: for example, some require organic additives, others do not work without expensive metals, some are far from environmentally friendly, and most are not flexible enough for wide use in a variety of applications. The Japanese team has now reported a new, highly promising ozone trap: two-line ferrihydrite, a mineral composed of iron, oxygen, and water. The researchers produce it by a self-assembly process using various additives and solvents. The resulting highly porous material consists of tiny aggregated nanocrystals and its name is derived from the fact that when examined by X-ray diffraction, a technique for structure analysis, it shows two lines.
Why is two-line ferrihydrite such a good ozone catcher? Thanks to its pores and arrangement of nanoparticles, this material has a large and accessible inner surface. In comparison to other iron oxide materials, it has a particularly high proportion of unsaturated and thus reactive iron centers on its surface. These have fewer oxygen atoms as near neighbors than the iron atoms in the interior of the mineral. Such isolated iron oxide units absorb ozone especially well. Once bound to the iron, the ozone splits into an oxygen molecule and a highly reactive oxygen atom, which can react with a second lone oxygen atom to form O2.
This material could replace conventional ozone filters in electrostatic devices and aircraft applications. It can also be used for air-cleaner devices or as a filter in ventilation or air-conditioning ducts in offices and houses. In ozone processes for water cleaning and odor removal, e.g. smoke from vehicle interiors, it could catch the unreacted O3.
- Mesoporous Ferrihydrite-Based Iron Oxide Nanoparticles as Highly Promising Materials for Ozone Removal,
Angew. Chem. Int. Ed. 2011, 50 (32).
DOI: 10.1002/anie.201102007