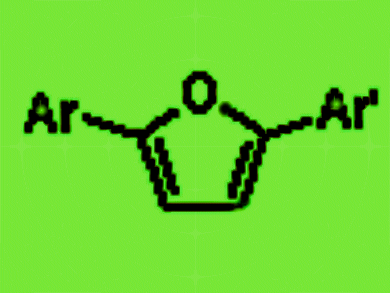
2-Substituted and 2,5-disubstituted furans are important substructures of several drugs currently in clinical use.
Bernd Schmidt and Diana Geißler, Universität Potsdam, Golm, Germany, have established a one-flask method for the synthesis of furans, ultimately starting from simple diallyl ethers. The sequence consists of transition-metal-catalyzed C–C bond-forming reactions, such as Ring-Closing Metathesis (RCM) and the Heck reaction, and subsequent oxidative aromatization through the use either of chloranil or of 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ).
In particular, the method is well suited for the synthesis of 2,5-disubstituted furans, including unsymmetrical 2,5-diaryl furans, which play an increasingly important role in medicinal chemistry and drug discovery.
- Ru- and Pd-Catalysed Synthesis of 2-Arylfurans by One-Flask Heck Arylation/Oxidation,
Bernd Schmidt, Diana Geißler,
Eur. J. Org. Chem. 2011.
DOI: 10.1002/ejoc.201100549